Quality of horse F(ab')2 antitoxins and anti-rabies immunoglobulins: protein content and anticomplementary activity
- PMID: 29946337
- PMCID: PMC6006770
- DOI: 10.1186/s40409-018-0153-z
Quality of horse F(ab')2 antitoxins and anti-rabies immunoglobulins: protein content and anticomplementary activity
Abstract
Background: Among other applications, immunotherapy is used for the post-exposure treatment and/or prophylaxis of important infectious diseases, such as botulism, diphtheria, tetanus and rabies. The effectiveness of serum therapy is widely proven, but improvements on the immunoglobulin purification process and on the quality control are necessary to reduce the amount of protein aggregates. These may trigger adverse reactions in patients by activating the complement system and inducing the generation of anaphylatoxins. Herein, we used immunochemical methods to predict the quality of horse F(ab')2 anti-botulinum AB, anti-diphtheric, antitetanic and anti-rabies immunoglobulins, in terms of amount of proteins and protein aggregates.
Methods: Samples were submitted to protein quantification, SDS-PAGE, Western blot analysis and molecular exclusion chromatography. The anticomplementary activity was determined in vitro by detecting the production of C5a/C5a desArg, the most potent anaphylatoxin. Data were analyzed by one-way ANOVA followed by Tukey's post-test, and differences were considered statistically significant when p < 0.05.
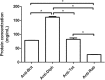
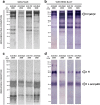
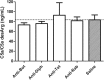
Results: Horse F(ab')2 antitoxins and anti-rabies immunoglobulin preparations presented different amounts of protein. SDS-PAGE and Western blot analyses revealed the presence of protein aggregates, non-immunoglobulin contaminants and, unexpectedly, IgG whole molecules in the samples, indicating the non-complete digestion of immunoglobulins. The chromatographic profiles of antitoxins and anti-rabies immunoglobulins allowed to estimate the percentage of contaminants and aggregates in the samples. Although protein aggregates were present, the samples were not able to induce the generation of C5a/C5a desArg in vitro, indicating that they probably contain acceptable levels of aggregates.
Conclusions: Anti-botulinum AB (bivalent), anti-diphtheric, antitetanic and anti-rabies horse F(ab')2 immunoglobulins probably contain acceptable levels of aggregates, although other improvements on the preparations must be carried out. Protein profile analysis and in vitro anticomplementary activity of F(ab')2 immunoglobulin preparations should be included as quality control steps, to ensure acceptable levels of aggregates, contaminants and whole IgG molecules on final products, reducing the chances of adverse reactions in patients.
Keywords: Anti-rabies; Antitoxins; Complement system; F(ab’)2 fragment; Heterologous immunoglobulin; Protein profile.
Conflict of interest statement
This study and the respective informed consent form were approved by the National Commission on Research Ethics – Research Ethics Committee of the Albert Einstein Hospital (CAAE02001612.6.0000.0071). Adult healthy donors were informed about the objectives of the study and signed the corresponding informed consent form.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Fundação Nacional de Saúde . 2ª Edição Revisada e Ampliada. 2000. Doenças infecciosas e parasitárias – aspectos clínicos, de vigilância epidemiológica e de controle – guia de bolso.
-
- World Health Organization: WHO guide for rabies pre and post exposure prophylaxis in humans. 2014. http://www.who.int/rabies/PEP_Prophylaxis_guideline_15_12_2014.pdf. Accessed 23 June 2017.
-
- Thwaites CL. Botulism and tetanus. Medicine. 2014;42(1):11–13. doi: 10.1016/j.mpmed.2013.10.013. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

