A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models
- PMID: 29946444
- PMCID: PMC6008850
- DOI: 10.12688/f1000research.14507.2
A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models
Abstract
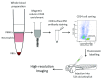
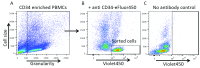
Haematopoietic stem cell (HSC) transplantation is a critical therapy for haematopoietic malignancies and immune disorders. Incomplete or delayed engraftment of HSCs in the host results in increased risk of infection and morbidity. The mechanisms of HSC engraftment are poorly understood and understanding these processes will increase transplantation success on many levels. Current animal models are immunocompromised 'humanised' mice transplanted with human HSCs. Harmful procedures include genetic manipulations and irradiation to ablate the mouse immune system, and opaque mouse tissues make visualisation of the early steps of HSC engraftment impossible. There is a need for new models to offer alternatives to humanised mice in the study of HSC transplantation. Here we described a detailed method for transplantation of human HSCs into zebrafish, before the onset of adaptive immunity. Human HSCs were purified from whole blood by enrichment of the CD34 cell population using a positive magnetic selection and further purified using an anti-CD34 antibody and cell sorting. Sorted CD34 cells were transplanted into the blood stream of 52 hour old zebrafish larvae. Human HSCs home into the zebrafish haematopoietic niche, where they engage with endothelial cells and undergo cell division. Our model offers the opportunities to image in vivo human HSC engraftment in a transparent organism, without the myeloablative strategies used in mice, and provides a unique system to understand the dynamic process of engraftment and replace current murine models. This technique can be applied to current engraftment protocols to validate the viability and efficiency of cryofrozen HSC grafts. This humanised zebrafish model will be instrumental to develop the 3Rs values in stem cell transplantation research and our detailed protocol will increase the chances of uptake of this zebrafish model by the mouse community.
Keywords: humanised zebrafish; stem cell transplantation; xenograft; zebrafish.
Conflict of interest statement
No competing interests were disclosed.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- NC/M001490/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- MR/M004864/1/MRC_/Medical Research Council/United Kingdom
- GR077544AIA/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G0700091/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

