Underlying Causes and Therapeutic Targeting of the Inflammatory Tumor Microenvironment
- PMID: 29946544
- PMCID: PMC6005853
- DOI: 10.3389/fcell.2018.00056
Underlying Causes and Therapeutic Targeting of the Inflammatory Tumor Microenvironment
Abstract
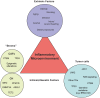
Historically, the link between chronic inflammation and cancer has long been speculated. Only more recently, pre-clinical and epidemiologic data as well as clinical evidence all point to the role of the tumor microenvironment as inextricably connected to the neoplastic process. The tumor microenvironment (TME), a complex mix of vasculature, inflammatory cells, and stromal cells is the essential "soil" helping to modulate tumor potential. Increasingly, evidence suggests that chronic inflammation modifies the tumor microenvironment, via a host of mechanisms, including the production of cytokines, pro-inflammatory mediators, angiogenesis, and tissue remodeling. Inflammation can be triggered by a variety of different pressures, such as carcinogen exposure, immune dysfunction, dietary habits, and obesity, as well as genetic alterations leading to oncogene activation or loss of tumor suppressors. In this review, we examine the concept of the tumor microenvironment as related to both extrinsic and intrinsic stimuli that promote chronic inflammation and in turn tumorigenesis. Understanding the common pathways inherent in an inflammatory response and the tumor microenvironment may shed light on new therapies for both primary and metastatic disease. The concept of personalized medicine has pushed the field of oncology to drill down on the genetic changes of a cancer, in the hopes of identifying individually targeted agents. Given the complexities of the tumor microenvironment, it is clear that effective oncologic therapies will necessitate targeting not only the cancer cells, but their dynamic relationship to the tumor microenvironment as well.
Keywords: anti-inflammatory drugs; chronic inflammation; clonal hematopoiesis; microenvironment; oncogenes; stroma; tumor suppressors.
Figures

References
-
- Agüera-González S., Burton O. T., Vázquez-Chávez E., Cuche C., Herit F., Bouchet J., et al. (2017). Adenomatous polyposis coli defines treg differentiation and anti-inflammatory function through microtubule-mediated NFAT localization. Cell Rep. 21, 181–194. 10.1016/j.celrep.2017.09.020 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

