Biochemical Properties of Human D-amino Acid Oxidase Variants and Their Potential Significance in Pathologies
- PMID: 29946548
- PMCID: PMC6005901
- DOI: 10.3389/fmolb.2018.00055
Biochemical Properties of Human D-amino Acid Oxidase Variants and Their Potential Significance in Pathologies
Abstract
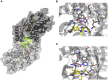
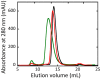
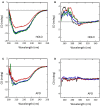
The stereoselective flavoenzyme D-amino acid oxidase (DAAO) catalyzes the oxidative deamination of neutral and polar D-amino acids producing the corresponding α-keto acids, ammonia, and hydrogen peroxide. Despite its peculiar and atypical substrates, DAAO is widespread expressed in most eukaryotic organisms. In mammals (and humans in particular), DAAO is involved in relevant physiological processes ranging from D-amino acid detoxification in kidney to neurotransmission in the central nervous system, where DAAO is responsible of the catabolism of D-serine, a key endogenous co-agonist of N-methyl-D-aspartate receptors. Recently, structural and functional studies have brought to the fore the distinctive biochemical properties of human DAAO (hDAAO). It appears to have evolved to allow a strict regulation of its activity, so that the enzyme can finely control the concentration of substrates (such as D-serine in the brain) without yielding to an excessive production of hydrogen peroxide, a potentially toxic reactive oxygen species (ROS). Indeed, dysregulation in D-serine metabolism, likely resulting from altered levels of hDAAO expression and activity, has been implicated in several pathologies, ranging from renal disease to neurological, neurodegenerative, and psychiatric disorders. Only one mutation in DAO gene was unequivocally associated to a human disease. However, several single nucleotide polymorphisms (SNPs) are reported in the database and the biochemical characterization of the corresponding recombinant hDAAO variants is of great interest for investigating the effect of mutations. Here we reviewed recently published data focusing on the modifications of the structural and functional properties induced by amino acid substitutions encoded by confirmed SNPs and on their effect on D-serine cellular levels. The potential significance of the different hDAAO variants in human pathologies will be also discussed.
Keywords: D-amino acid oxidase; D-amino acids; D-serine levels; human pathologies; protein conformation; protein variants; structural-functional properties.
Figures
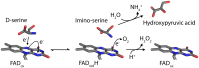




Similar articles
-
Structure-function relationships in human d-amino acid oxidase variants corresponding to known SNPs.Biochim Biophys Acta. 2015 Sep;1854(9):1150-9. doi: 10.1016/j.bbapap.2015.02.005. Epub 2015 Feb 17. Biochim Biophys Acta. 2015. PMID: 25701391
-
Biochemical Properties of Human D-Amino Acid Oxidase.Front Mol Biosci. 2017 Dec 15;4:88. doi: 10.3389/fmolb.2017.00088. eCollection 2017. Front Mol Biosci. 2017. PMID: 29326945 Free PMC article.
-
Human d-amino acid oxidase: The inactive G183R variant.Biochim Biophys Acta Proteins Proteom. 2018 Jul;1866(7):822-830. doi: 10.1016/j.bbapap.2017.12.007. Epub 2017 Dec 21. Biochim Biophys Acta Proteins Proteom. 2018. PMID: 29274788
-
Competitive Inhibitors Unveil Structure/Function Relationships in Human D-Amino Acid Oxidase.Front Mol Biosci. 2017 Nov 27;4:80. doi: 10.3389/fmolb.2017.00080. eCollection 2017. Front Mol Biosci. 2017. PMID: 29250527 Free PMC article. Review.
-
Human D-Amino Acid Oxidase: Structure, Function, and Regulation.Front Mol Biosci. 2018 Nov 28;5:107. doi: 10.3389/fmolb.2018.00107. eCollection 2018. Front Mol Biosci. 2018. PMID: 30547037 Free PMC article. Review.
Cited by
-
Mechanistic Multilayer Quantitative Model for Nonlinear Pharmacokinetics, Target Occupancy and Pharmacodynamics (PK/TO/PD) Relationship of D-Amino Acid Oxidase Inhibitor, TAK-831 in Mice.Pharm Res. 2020 Aug 5;37(8):164. doi: 10.1007/s11095-020-02893-x. Pharm Res. 2020. PMID: 32901384 Free PMC article.
-
Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease.Antioxidants (Basel). 2021 Dec 30;11(1):91. doi: 10.3390/antiox11010091. Antioxidants (Basel). 2021. PMID: 35052595 Free PMC article. Review.
-
Raising the 'Good' Oxidants for Immune Protection.Front Immunol. 2021 Jun 4;12:698042. doi: 10.3389/fimmu.2021.698042. eCollection 2021. Front Immunol. 2021. PMID: 34149739 Free PMC article. Review.
-
Phase Separation Regulates Metabolism, Mitochondria, and Diseases.MedComm (2020). 2025 Jul 1;6(7):e70283. doi: 10.1002/mco2.70283. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599234 Free PMC article. Review.
-
d-amino Acids in Health and Disease: A Focus on Cancer.Nutrients. 2019 Sep 12;11(9):2205. doi: 10.3390/nu11092205. Nutrients. 2019. PMID: 31547425 Free PMC article. Review.
References
-
- Adage T., Trillat A. C., Quattropani A., Perrin D., Cavarec L., Shaw J., et al. (2008). In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur. Neuropsychopharmacol. 18, 200–214. 10.1016/j.euroneuro.2007.06.006 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

