Patterns of Bicalutamide Use in Prostate Cancer Treatment: A U.S. Real-World Analysis Using the SEER-Medicare Database
- PMID: 29946798
- PMCID: PMC6133134
- DOI: 10.1007/s12325-018-0738-5
Patterns of Bicalutamide Use in Prostate Cancer Treatment: A U.S. Real-World Analysis Using the SEER-Medicare Database
Erratum in
-
Correction to: Patterns of Bicalutamide Use in Prostate Cancer Treatment: A U.S. Real-World Analysis Using the SEER-Medicare Database.Adv Ther. 2018 Sep;35(9):1452. doi: 10.1007/s12325-018-0767-0. Adv Ther. 2018. PMID: 30117060 Free PMC article.
Abstract
Introduction: Bicalutamide (BIC), a non-steroidal anti-androgen, is FDA-indicated for use in combination with a luteinizing hormone-releasing hormone (LHRH) analog for treatment of Stage D2 metastatic carcinoma of the prostate. Lack of consensus exists regarding the clinical benefit of BIC use, either alone or combined use of BIC with an LHRH analog or antagonist (combined androgen blockade or CAB), versus treatment with androgen deprivation therapy (ADT) alone.
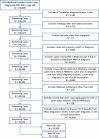
Methods: The SEER-Medicare database was used to identify prostate cancer patients aged ≥ 66 years diagnosed between 2007 and 2011 and who filled at least one prescription for BIC. Duration of BIC treatment was assessed in relation to ADT use; either alone (monotherapy), as part of CAB only, and as part of CAB followed by monotherapy. Additionally, we assessed use of BIC during or outside a potential testosterone flare prevention period (initiation within 2 months of an LHRH agonist).
Results: A total of 7521 prostate cancer patients who filled a prescription for BIC were identified. Eighteen percent of the cohort used BIC alone, over half the patients (54%) used BIC as part of CAB and 27% used BIC as part of CAB followed by monotherapy. Among men treated with BIC as part of CAB, 58% received BIC only within the potential flare period.
Conclusions: Although there is no FDA indication for BIC use as monotherapy, > 44% of patients in this study used BIC alone or as part of CAB followed by monotherapy. Further research is necessary to understand the outcomes of BIC utilization in these settings, particularly compared with newer second-generation anti-androgens.
Funding: Medivation LLC, a Pfizer company, and Astellas, Pharma, Inc.
Keywords: Bicalutamide; Non-steroidal anti-androgen; Prostate cancer; Treatment patterns.
Conflict of interest statement
Jennifer L. Beebe-Dimmer is an employee of Wayne State University, which received a grant from EpidStat for their contributions. Julie J. Ruterbusch is an employee of Wayne State University, which received a grant from EpidStat for their contributions. Lauren C. Bylsma is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Christina Gillezeau is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Jon Fryzek is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Scott C. Flanders holds stock in Johnson & Johnson, Abbott Laboratories, and Abbvie. Ruben G. W. Quek received remuneration and funding from Pfizer for this work and holds stock in Pfizer and Amgen. Neil M. Schultz, Arie Barlev and Elisabeth Heath have no conflicts of interest to disclose.
Figures
References
-
- Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–63. 10.1016/S1470-2045(15)00518-5 - DOI - PubMed
-
- Senagore AJ. The gale encyclopedia of surgery: G-O, vol. 2. Detroit, MI: Gale Group; 2004. pp. 1051–58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical