The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin
- PMID: 29947252
- PMCID: PMC6158549
- DOI: 10.1177/0963689718779346
The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin
Abstract
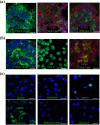
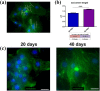
Ischemic heart disease, also known as coronary artery disease (CAD), poses a challenge for regenerative medicine. iPSC technology might lead to a breakthrough due to the possibility of directed cell differentiation delivering a new powerful source of human autologous cardiomyocytes. One of the factors supporting proper cell maturation is in vitro culture duration. In this study, primary human skeletal muscle myoblasts were selected as a myogenic cell type reservoir for genetic iPSC reprogramming. Skeletal muscle myoblasts have similar ontogeny embryogenetic pathways (myoblasts vs. cardiomyocytes), and thus, a greater chance of myocardial development might be expected, with maintenance of acquired myogenic cardiac cell characteristics, from the differentiation process when iPSCs of myoblastoid origin are obtained. Analyses of cell morphological and structural changes, gene expression (cardiac markers), and functional tests (intracellular calcium transients) performed at two in vitro culture time points spanning the early stages of cardiac development (day 20 versus 40 of cell in vitro culture) confirmed the ability of the obtained myogenic cells to acquire adult features of differentiated cardiomyocytes. Prolonged 40-day iPSC-derived cardiomyocytes (iPSC-CMs) revealed progressive cellular hypertrophy; a better-developed contractile apparatus; expression of marker genes similar to human myocardial ventricular cells, including a statistically significant CX43 increase, an MHC isoform switch, and a troponin I isoform transition; more efficient intercellular calcium handling; and a stronger response to β-adrenergic stimulation.
Keywords: Cardiac differentiation; cardiomyocyte maturation; cardiomyogenesis; iPSCs; skeletal myoblasts.
Conflict of interest statement
Figures













References
-
- Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28(2):208–16. - PubMed
-
- Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rücker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagège A, Desnos M, Renaud J-F, Menasché P, Pucéat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–39. - PMC - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. - PubMed
-
- Thomson JA, Shapiro SS, Waknitz MA, Marshall VS. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

