Synthesis of hydrophobic insulin-based peptides using a helping hand strategy
- PMID: 29947407
- PMCID: PMC6310105
- DOI: 10.1039/c8ob01212a
Synthesis of hydrophobic insulin-based peptides using a helping hand strategy
Abstract
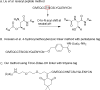
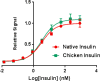
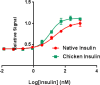
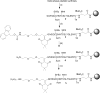
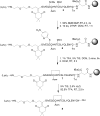
The introduction of solid-phase peptide synthesis in the 1960s improved the chemical synthesis of both the A- and B-chains of insulin and insulin analogs. However, the subsequent elaboration of the synthetic peptides to generate active hormones continues to be difficult and complex due in part to the hydrophobicity of the A-chain. Over the past decade, several groups have developed different methods to enhance A-chain solubility. Two of the most popular methods are use of isoacyl dipeptides, and the attachment of an A-chain C-terminal pentalysine tag with a base-labile 4-hydroxymethylbenzoic acid linker. These methods have proven effective but can be limited in scope depending on the peptide sequence of a specific insulin. Herein we describe an auxiliary approach to enhance the solubility of insulin-based peptides by incorporating a tri-lysine tag attached to a cleavable Fmoc-Ddae-OH linker. Incorporation of this linker, or "helping hand", on the N-terminus greatly improved the solubility of chicken insulin A-chain, which is analogous to human insulin, and allowed for coupling of the insulin A- and B-chain via directed disulfide bond formation. After formation of the insulin heterodimer, the linker and tag could be easily removed using a hydrazine buffer (pH 7.5) to obtain an overall 12.6% yield based on A-chain. This strategy offers an efficient method to enhance the solubility of hydrophobic insulin-based peptides as well as other traditionally difficult peptides.
Conflict of interest statement
There are no conflicts to declare.
Figures
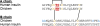
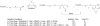


Similar articles
-
Use of a temporary "solubilizing" peptide tag for the Fmoc solid-phase synthesis of human insulin glargine via use of regioselective disulfide bond formation.Bioconjug Chem. 2009 Jul;20(7):1390-6. doi: 10.1021/bc900181a. Bioconjug Chem. 2009. PMID: 19552405
-
A Helping Hand to Overcome Solubility Challenges in Chemical Protein Synthesis.J Am Chem Soc. 2016 Sep 14;138(36):11775-82. doi: 10.1021/jacs.6b05719. Epub 2016 Sep 1. J Am Chem Soc. 2016. PMID: 27532670 Free PMC article.
-
Chemical Synthesis of Human Insulin-Like Peptide-6.Chemistry. 2016 Jul 4;22(28):9777-83. doi: 10.1002/chem.201601410. Epub 2016 Jun 3. Chemistry. 2016. PMID: 27259101
-
Chemical synthesis of peptides within the insulin superfamily.J Pept Sci. 2016 May;22(5):260-70. doi: 10.1002/psc.2863. Epub 2016 Feb 22. J Pept Sci. 2016. PMID: 26910514 Review.
-
Handles for Fmoc solid-phase synthesis of protected peptides.ACS Comb Sci. 2013 May 13;15(5):217-28. doi: 10.1021/co300153c. Epub 2013 Apr 26. ACS Comb Sci. 2013. PMID: 23573835 Review.
Cited by
-
A cysteine-specific solubilizing tag strategy enables efficient chemical protein synthesis of difficult targets.Chem Sci. 2024 Jan 19;15(9):3214-3222. doi: 10.1039/d3sc06032b. eCollection 2024 Feb 28. Chem Sci. 2024. PMID: 38425513 Free PMC article.
-
Single-site glycine-specific labeling of proteins.Nat Commun. 2019 Jun 10;10(1):2539. doi: 10.1038/s41467-019-10503-7. Nat Commun. 2019. PMID: 31182711 Free PMC article.
-
Improved Handling of Peptide Segments Using Side Chain-Based "Helping Hand" Solubilizing Tools.Methods Mol Biol. 2022;2530:81-107. doi: 10.1007/978-1-0716-2489-0_7. Methods Mol Biol. 2022. PMID: 35761044
-
Challenges and Perspectives in Chemical Synthesis of Highly Hydrophobic Peptides.Front Bioeng Biotechnol. 2020 Mar 4;8:162. doi: 10.3389/fbioe.2020.00162. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32195241 Free PMC article. Review.
-
D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities.Chembiochem. 2023 Feb 14;24(4):e202200537. doi: 10.1002/cbic.202200537. Epub 2022 Nov 16. Chembiochem. 2023. PMID: 36278392 Free PMC article. Review.
References
-
- Merrifield RB. Journal of the American Chemical Society. 1963;85:2149–2154.
-
- Kung YT, Du YC, Huang WT, Chen CC, Ke LT. Sci Sin. 1965;14:1710–1716. - PubMed
-
- Katsoyannis PG, Tometsko A, Zalut C. J Am Chem Soc. 1966;88:166–167. - PubMed
-
- Marglin A, Merrifield RB. J Am Chem Soc. 1966;88:5051–5052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

