Do HIV treatment eligibility expansions crowd out the sickest? Evidence from rural South Africa
- PMID: 29947442
- PMCID: PMC6175239
- DOI: 10.1111/tmi.13122
Do HIV treatment eligibility expansions crowd out the sickest? Evidence from rural South Africa
Abstract
Objective: The 2015 WHO recommendation to initiate all HIV patients on antiretroviral therapy (ART) at diagnosis could potentially overextend health systems and crowd out sicker patients, mitigating the policy's impact. We evaluate whether South Africa's prior eligibility expansion from CD4 ≤ 200 to CD4 ≤ 350 cells/μl reduced ART uptake in the sickest patients.
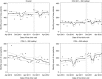
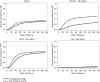
Methods: Using data on all patients presenting to the Hlabisa HIV Treatment and Care Programme in KwaZulu-Natal from April 2010 to June 2012 (n = 13 809), we assessed the impact of the August 2011 eligibility expansion on the number of patients seeking care, number initiating ART and time from HIV diagnosis to ART initiation among patients always eligible (CD4 0-200), newly eligible (CD4 201-350) and not yet eligible by CD4 count (>350). We used interrupted time series methods to control for long-run trends and isolate the effect of the policy.
Results: Expanding ART eligibility led to an increased number of patients initiating ART per month [+95.5; 95% CI (-1.3; 192.3)]. Newly eligible patients (CD4 201-350) initiated treatment 47% faster than before (95% CI 19%; 82%), while the sickest patients (CD4 ≤ 200) saw no decline in the monthly number of patients initiating treatment or the rate of treatment uptake.
Conclusion: The Hlabisa programme successfully extended ART to patients with CD4 ≤ 350 cells/μl, while ensuring that the sickest patients did not experience delays in ART initiation. Treatment programmes must be vigilant to maintain quality of care for the sickest as countries move to treat all patients irrespective of CD4 count.
Objectif: La recommandation 2015 de l'OMS d'initier tous les patients VIH à la thérapie antirétrovirale (ART) dès le diagnostic pourrait potentiellement surpasser les systèmes de santé et évincer les patients plus malades, atténuant ainsi l'impact de la politique. Nous avons voulu évaluer si l'expansion préalable de l’éligibilité en l'Afrique du Sud, passant de CD4 ≤ 200 à CD4 ≤350 cellules/μL, réduisait l'adoption de l'ART chez les patients les plus malades.
Méthodes: En utilisant des données sur tous les patients du programme Hlabisa de traitement et de soins du VIH dans le KwaZulu‐Natal d'avril 2010 à juin 2012 (n = 13 809), nous avons évalué l'impact de l'expansion de l’éligibilité en août 2011 sur le nombre de patients recherchant des soins, le nombre débutant l'ART et le temps écoulé entre le diagnostic du VIH et le début de l'ART parmi les patients toujours éligibles (CD4<200), nouvellement éligibles (201<CD4<350) et non encore éligibles (CD4>350). Nous avons utilisé des méthodes de séries temporelles interrompues pour contrôler pour les tendances à long terme et isoler l'effet de la politique.
Résultats: L'expansion de l’éligibilité a entraîné une augmentation du nombre de patients débutant l'ART [+95.5; IC95% (−1.3; 192.3)]. Les patients nouvellement éligibles (201 < CD4 < 350) ont débuté le traitement 47% plus vite qu'avant (IC95%: 19%; 82%), tandis que les patients les plus malades (CD4 ≤200) n'ont pas vu diminuer le nombre mensuel débutant le traitement ou le taux de l'adoption du traitement.
Conclusion: Le programme Hlabisa a étendu l'ART avec succès chez les patients avec CD4 ≤350 cellules/μL tout en veillant à ce que les patients les plus malades ne subissent pas de retards dans l'initiation de l'ART. Les programmes de traitement doivent être vigilants afin de maintenir la qualité des soins pour les personnes les plus malades à mesure que les pays commencent à traiter tous les patients sans tenir compte du nombre de CD4.
Keywords: Afrique du Sud; HIV/AIDS; South Africa; adultes; adults; antiretroviral therapy; continuity of care; continuité des soins; directives; guidelines; thérapie antirétrovirale.
© 2018 The Authors. Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Failure to initiate HIV treatment in patients with high CD4 counts: evidence from demographic surveillance in rural South Africa.Trop Med Int Health. 2018 Feb;23(2):206-220. doi: 10.1111/tmi.13013. Epub 2018 Jan 10. Trop Med Int Health. 2018. PMID: 29160949 Free PMC article.
-
Continuum in HIV care from entry to ART initiation in rural KwaZulu-Natal, South Africa.Trop Med Int Health. 2014 Jun;19(6):680-689. doi: 10.1111/tmi.12301. Epub 2014 Mar 21. Trop Med Int Health. 2014. PMID: 24654990
-
HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries.PLoS Med. 2018 Mar 23;15(3):e1002534. doi: 10.1371/journal.pmed.1002534. eCollection 2018 Mar. PLoS Med. 2018. PMID: 29570723 Free PMC article.
-
Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa.PLoS Med. 2017 Nov 28;14(11):e1002463. doi: 10.1371/journal.pmed.1002463. eCollection 2017 Nov. PLoS Med. 2017. PMID: 29182641 Free PMC article.
-
Effect of eliminating CD4-count thresholds on HIV treatment initiation in South Africa: An empirical modeling study.PLoS One. 2017 Jun 15;12(6):e0178249. doi: 10.1371/journal.pone.0178249. eCollection 2017. PLoS One. 2017. PMID: 28617805 Free PMC article.
Cited by
-
Failure to initiate HIV treatment in patients with high CD4 counts: evidence from demographic surveillance in rural South Africa.Trop Med Int Health. 2018 Feb;23(2):206-220. doi: 10.1111/tmi.13013. Epub 2018 Jan 10. Trop Med Int Health. 2018. PMID: 29160949 Free PMC article.
-
Early outcomes after implementation of treat all in Rwanda: an interrupted time series study.J Int AIDS Soc. 2019 Apr;22(4):e25279. doi: 10.1002/jia2.25279. J Int AIDS Soc. 2019. PMID: 30993854 Free PMC article.
-
Changes in rapid HIV treatment initiation after national "treat all" policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis.PLoS Med. 2019 Jun 10;16(6):e1002822. doi: 10.1371/journal.pmed.1002822. eCollection 2019 Jun. PLoS Med. 2019. PMID: 31181056 Free PMC article.
-
Cohort profile: the South African National Health Laboratory Service (NHLS) National HIV Cohort.BMJ Open. 2022 Oct 19;12(10):e066671. doi: 10.1136/bmjopen-2022-066671. BMJ Open. 2022. PMID: 36261238 Free PMC article.
-
Predictors of antiretroviral therapy initiation in eThekwini (Durban), South Africa: Findings from a prospective cohort study.PLoS One. 2021 Feb 19;16(2):e0246744. doi: 10.1371/journal.pone.0246744. eCollection 2021. PLoS One. 2021. PMID: 33606712 Free PMC article.
References
-
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013 [Internet]. Geneva, Switzerland: World Health Organization; 2013. (Available from: http://www.who.int/hiv/pub/guidelines/arv2013/art/statartadolescents/en/) [13 May 2014]. - PubMed
-
- Danel C, Moh R, Gabillard D et al A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015: 373: 808–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

