Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core
- PMID: 29947588
- PMCID: PMC6171053
- DOI: 10.1152/jn.00263.2018
Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core
Abstract
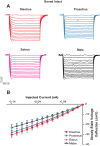
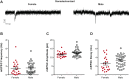
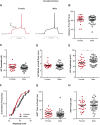
Naturally occurring hormone cycles in adult female humans and rodents create a dynamic neuroendocrine environment. These cycles include the menstrual cycle in humans and its counterpart in rodents, the estrous cycle. These hormone fluctuations induce sex differences in the phenotypes of many behaviors, including those related to motivation, and associated disorders such as depression and addiction. This suggests that the neural substrate instrumental for these behaviors, including the nucleus accumbens core (AcbC), likewise differs between estrous cycle phases. It is unknown whether the electrophysiological properties of AcbC output neurons, medium spiny neurons (MSNs), change between estrous cycle phases. This is a critical knowledge gap given that MSN electrophysiological properties are instrumental for determining AcbC output to efferent targets. Here we test whether the intrinsic electrophysiological properties of adult rat AcbC MSNs differ across female estrous cycle phases and from males. We recorded MSNs with whole cell patch-clamp technique in two experiments, the first using gonad-intact adult males and females in differing phases of the estrous cycle and the second using gonadectomized males and females in which the estrous cycle was eliminated. MSN intrinsic electrophysiological and excitatory synaptic input properties robustly changed between female estrous cycle phases and males. Sex differences in MSN electrophysiology disappeared when the estrous cycle was eliminated. These novel findings indicate that AcbC MSN electrophysiological properties change across the estrous cycle, providing a new framework for understanding how biological sex and hormone cyclicity regulate motivated behaviors and other AcbC functions and disorders. NEW & NOTEWORTHY This research is the first demonstration that medium spiny neuron electrophysiological properties change across adult female hormone cycle phases in any striatal region. This influence of estrous cycle engenders sex differences in electrophysiological properties that are eliminated by gonadectomy. Broadly, these findings indicate that adult female hormone cycles are an important factor for neurophysiology.
Keywords: estrous cycle; excitability; medium spiny neurons; nucleus accumbens; sex steroid hormones.
Figures







Similar articles
-
Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology.J Neurophysiol. 2020 Jun 1;123(6):2390-2405. doi: 10.1152/jn.00157.2020. Epub 2020 May 13. J Neurophysiol. 2020. PMID: 32401164 Free PMC article.
-
Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice.J Neurophysiol. 2018 Oct 1;120(4):1712-1727. doi: 10.1152/jn.00257.2018. Epub 2018 Jul 5. J Neurophysiol. 2018. PMID: 29975170 Free PMC article.
-
Estradiol decreases medium spiny neuron excitability in female rat nucleus accumbens core.J Neurophysiol. 2020 Jun 1;123(6):2465-2475. doi: 10.1152/jn.00210.2020. Epub 2020 May 20. J Neurophysiol. 2020. PMID: 32432511 Free PMC article.
-
The estrous cycle and 17β-estradiol modulate the electrophysiological properties of rat nucleus accumbens core medium spiny neurons.J Neuroendocrinol. 2022 Jun;34(6):e13122. doi: 10.1111/jne.13122. Epub 2022 Apr 2. J Neuroendocrinol. 2022. PMID: 35365910 Free PMC article. Review.
-
Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation.Front Endocrinol (Lausanne). 2018 Apr 18;9:173. doi: 10.3389/fendo.2018.00173. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29720962 Free PMC article. Review.
Cited by
-
Metabotropic glutamate receptor subtype 5 (mGlu5) is necessary for estradiol mitigation of light-induced anxiety behavior in female rats.Physiol Behav. 2020 Feb 1;214:112770. doi: 10.1016/j.physbeh.2019.112770. Epub 2019 Dec 9. Physiol Behav. 2020. PMID: 31830486 Free PMC article.
-
Cocaine-induced sensitization and glutamate plasticity in the nucleus accumbens core: effects of sex.Biol Sex Differ. 2023 Jun 24;14(1):41. doi: 10.1186/s13293-023-00525-8. Biol Sex Differ. 2023. PMID: 37355656 Free PMC article.
-
Sexual Differentiation and Substance Use: A Mini-Review.Endocrinology. 2020 Sep 1;161(9):bqaa129. doi: 10.1210/endocr/bqaa129. Endocrinology. 2020. PMID: 32761086 Free PMC article. Review.
-
Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology.J Neurophysiol. 2020 Jun 1;123(6):2390-2405. doi: 10.1152/jn.00157.2020. Epub 2020 May 13. J Neurophysiol. 2020. PMID: 32401164 Free PMC article.
-
Fundamental Sex Differences in Cocaine-Induced Plasticity of Dopamine D1 Receptor- and D2 Receptor-Expressing Medium Spiny Neurons in the Mouse Nucleus Accumbens Shell.Biol Psychiatry Glob Open Sci. 2024 Feb 19;4(3):100295. doi: 10.1016/j.bpsgos.2024.100295. eCollection 2024 May. Biol Psychiatry Glob Open Sci. 2024. PMID: 38533248 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

