The stringent response and Mycobacterium tuberculosis pathogenesis
- PMID: 29947752
- PMCID: PMC7191866
- DOI: 10.1093/femspd/fty054
The stringent response and Mycobacterium tuberculosis pathogenesis
Abstract
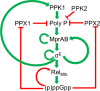
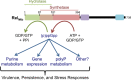
During infection, the host restrains Mycobacterium tuberculosis (Mtb) from proliferating by imposing an arsenal of stresses. Despite this onslaught of attacks, Mtb is able to persist for the lifetime of the host, indicating that this pathogen has substantial molecular mechanisms to resist host-inflicted damage. The stringent response is a conserved global stress response in bacteria that involves the production of the hyperphosphorylated guanine nucleotides ppGpp and pppGpp (collectively called (p)ppGpp). (p)ppGpp then regulates a number of cellular processes to adjust the physiology of the bacteria to promote survival in different environments. Survival in the presence of host-generated stresses is an essential quality of successful pathogens, and the stringent response is critical for the intracellular survival of a number of pathogenic bacteria. In addition, the stringent response has been linked to virulence gene expression, persistence, latency and drug tolerance. In Mtb, (p)ppGpp synthesis is required for survival in low nutrient conditions, long term culture and during chronic infection in animal models, all indicative of a strict requirement for (p)ppGpp during exposure to stresses associated with infection. In this review we discuss (p)ppGpp metabolism and how this functions as a critical regulator of Mtb virulence.
Figures

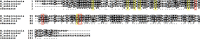
References
-
- Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 1998;23:469–72. - PubMed
-
- Avarbock A, Avarbock D, Teh J-S et al. Functional regulation of the opposing (p)ppGpp synthetase/hydrolase activities of RelMtb from Mycobacterium tuberculosis. Biochemistry 2005;44:9913–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

