Cardioplegia in paediatric cardiac surgery: a systematic review of randomized controlled trials
- PMID: 29947787
- PMCID: PMC6328004
- DOI: 10.1093/icvts/ivy199
Cardioplegia in paediatric cardiac surgery: a systematic review of randomized controlled trials
Abstract
Objectives: Cardioplegia is the primary method for myocardial protection during cardiac surgery. We conducted a systematic review of randomized controlled trials of cardioplegia in children to evaluate the current evidence base.
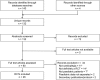
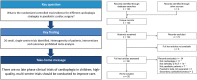
Methods: We searched MEDLINE, CENTRAL and LILACS and manually screened retrieved references and systematic reviews to identify all randomized controlled trials comparing cardioplegia solutions or additives in children undergoing cardiac surgery published in any language; secondary publications and those reporting inseparable adult data were excluded. Two or more reviewers independently screened studies for eligibility and extracted data; the Cochrane Risk of Bias tool was used to assess for potential biases.
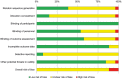
Results: We identified 26 trials randomizing 1596 children undergoing surgery; all were single-centre, Phase II trials, recruiting few patients (median 48, interquartile range 30-99). The most frequent comparison was blood versus crystalloid in 10 (38.5%) trials, and the most common end points were biomarkers of myocardial injury (17, 65.4%), inotrope requirements (15, 57.7%) and length of stay in the intensive care unit (11, 42.3%). However, the heterogeneity of patients, interventions and reported outcome measures prohibited meta-analysis. Overall risk of bias was high in 3 (11.5%) trials, unclear in 23 (88.5%) and low in none.
Conclusions: The current literature on cardioplegia in children contains no late phase trials. The small size, inconsistent use of end points and low quality of reported trials provide a limited evidence base to inform practice. A core outcome set of clinically important, standardized, validated end points for assessing myocardial protection in children should be developed to facilitate the conduct of high-quality, multicentre trials.
Prospero registration: CRD42017080205.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Randomized controlled trials in children's heart surgery in the 21st century: a systematic review.Eur J Cardiothorac Surg. 2018 Apr 1;53(4):724-731. doi: 10.1093/ejcts/ezx388. Eur J Cardiothorac Surg. 2018. PMID: 29186478 Free PMC article.
-
Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials.Circulation. 2006 Jul 4;114(1 Suppl):I331-8. doi: 10.1161/CIRCULATIONAHA.105.001644. Circulation. 2006. PMID: 16820596
-
del Nido versus St. Thomas' blood cardioplegia in the young (DESTINY) trial: protocol for a multicentre randomised controlled trial in children undergoing cardiac surgery.BMJ Open. 2025 Apr 14;15(4):e102029. doi: 10.1136/bmjopen-2025-102029. BMJ Open. 2025. PMID: 40228861 Free PMC article.
-
Cold blood versus crystalloid cardioplegia for myocardial protection in adult cardiac surgery: a meta-analysis of randomized controlled studies.J Cardiothorac Vasc Anesth. 2014 Jun;28(3):674-81. doi: 10.1053/j.jvca.2013.06.005. Epub 2014 Apr 12. J Cardiothorac Vasc Anesth. 2014. PMID: 24721161 Review.
Cited by
-
Myocardial protection in paediatric cardiac surgery: building an evidence-based strategy.Ann R Coll Surg Engl. 2024 Mar;106(3):277-282. doi: 10.1308/rcsann.2023.0004. Epub 2023 May 30. Ann R Coll Surg Engl. 2024. PMID: 37249560 Free PMC article.
-
International Pediatric Perfusion Practice: 2016 Survey Results.J Extra Corpor Technol. 2021 Mar;53(1):7-26. doi: 10.1182/ject-2000033. J Extra Corpor Technol. 2021. PMID: 33814602 Free PMC article.
-
Custodiol versus blood cardioplegia in pediatric cardiac surgery: a randomized controlled trial.Eur J Med Res. 2023 Oct 5;28(1):404. doi: 10.1186/s40001-023-01372-4. Eur J Med Res. 2023. PMID: 37798628 Free PMC article. Clinical Trial.
-
Cardioplegia practice in paediatric cardiac surgery: a UK & Ireland survey.Perfusion. 2019 Mar;34(2):125-129. doi: 10.1177/0267659118794343. Epub 2018 Aug 10. Perfusion. 2019. PMID: 30095360 Free PMC article. Clinical Trial.
-
Intermittent antegrade warm-blood versus cold-blood cardioplegia in children undergoing open heart surgery: a protocol for a randomised controlled study (Thermic-3).BMJ Open. 2020 Oct 14;10(10):e036974. doi: 10.1136/bmjopen-2020-036974. BMJ Open. 2020. PMID: 33055113 Free PMC article.
References
-
- Buckberg GD, Brazier JR, Nelson RL, Goldstein SM, McConnell DH, Cooper N.. Studies of the effects of hypothermia on regional myocardial blood flow and metabolism during cardiopulmonary bypass. I. The adequately perfused beating, fibrillating and arrested heart. J Thorac Cardiovasc Surg 1977;73:87–94. - PubMed
-
- Mildh LH, Pettilä V, Sairanen HI, Rautiainen PH.. Cardiac troponin T levels for risk stratification in pediatric open heart surgery. Ann Thorac Surg 2006;82:1643–9. - PubMed
-
- Chaturvedi RR, Lincoln C, Gothard JW, Scallan MH, White PA, Redington AN. et al. Left ventricular dysfunction after open repair of simple congenital heart defects in infants and children: quantification with the use of a conductance catheter immediately after bypass. J Thorac Cardiovasc Surg 1998;115:77–83. - PubMed
-
- Doenst T, Schlensak C, Beyersdorf F.. Cardioplegia in pediatric cardiac surgery: do we believe in magic? Ann Thorac Surg 2003;75:1668–77. - PubMed
-
- del Nido PJ, Mickle DA, Wilson GJ, Benson LN, Weisel RD, Coles JG. et al. Inadequate myocardial protection with cold cardioplegia arrest during repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 1988;95:223–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical