The HDAC6/8/10 inhibitor TH34 induces DNA damage-mediated cell death in human high-grade neuroblastoma cell lines
- PMID: 29947893
- PMCID: PMC6063332
- DOI: 10.1007/s00204-018-2234-8
The HDAC6/8/10 inhibitor TH34 induces DNA damage-mediated cell death in human high-grade neuroblastoma cell lines
Abstract
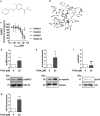
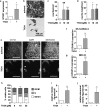
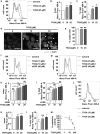
High histone deacetylase (HDAC) 8 and HDAC10 expression levels have been identified as predictors of exceptionally poor outcomes in neuroblastoma, the most common extracranial solid tumor in childhood. HDAC8 inhibition synergizes with retinoic acid treatment to induce neuroblast maturation in vitro and to inhibit neuroblastoma xenograft growth in vivo. HDAC10 inhibition increases intracellular accumulation of chemotherapeutics through interference with lysosomal homeostasis, ultimately leading to cell death in cultured neuroblastoma cells. So far, no HDAC inhibitor covering HDAC8 and HDAC10 at micromolar concentrations without inhibiting HDACs 1, 2 and 3 has been described. Here, we introduce TH34 (3-(N-benzylamino)-4-methylbenzhydroxamic acid), a novel HDAC6/8/10 inhibitor for neuroblastoma therapy. TH34 is well-tolerated by non-transformed human skin fibroblasts at concentrations up to 25 µM and modestly impairs colony growth in medulloblastoma cell lines, but specifically induces caspase-dependent programmed cell death in a concentration-dependent manner in several human neuroblastoma cell lines. In addition to the induction of DNA double-strand breaks, HDAC6/8/10 inhibition also leads to mitotic aberrations and cell-cycle arrest. Neuroblastoma cells display elevated levels of neuronal differentiation markers, mirrored by formation of neurite-like outgrowths under maintained TH34 treatment. Eventually, after long-term treatment, all neuroblastoma cells undergo cell death. The combination of TH34 with plasma-achievable concentrations of retinoic acid, a drug applied in neuroblastoma therapy, synergistically inhibits colony growth (combination index (CI) < 0.1 for 10 µM of each). In summary, our study supports using selective HDAC inhibitors as targeted antineoplastic agents and underlines the therapeutic potential of selective HDAC6/8/10 inhibition in high-grade neuroblastoma.
Keywords: DNA repair; Differentiation; HDAC10; HDAC8; Selective histone deacetylase inhibitor; Targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Adamson PC. All-trans-retinoic acid pharmacology and its impact on the treatment of acute promyelocytic leukemia. Oncologist. 1996;1(5):305–314. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

