Coordination of microtubule acetylation and the actin cytoskeleton by formins
- PMID: 29947928
- PMCID: PMC11105221
- DOI: 10.1007/s00018-018-2855-3
Coordination of microtubule acetylation and the actin cytoskeleton by formins
Abstract
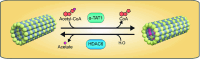
The acetylation of the lysine 40 residue of α-tubulin was described more than 30 years ago and has been the subject of intense research ever since. Although the exact function of this covalent modification of tubulin in the cell remains unknown, it has been established that tubulin acetylation confers resilience to mechanical stress on the microtubules. Formins have a dual role in the fate of the actin and tubulin cytoskeletons. On the one hand, they catalyze the formation of actin filaments, and on the other, they bind microtubules, act on their stability, and regulate their acetylation and alignment with actin fibers. Recent evidence indicates that formins coordinate the actin cytoskeleton and tubulin acetylation by modulating the levels of free globular actin (G-actin). G-actin, in turn, controls the activity of the myocardin-related transcription factor-serum response factor transcriptional complex that regulates the expression of the α-tubulin acetyltransferase 1 (α-TAT1) gene, which encodes the main enzyme responsible for tubulin acetylation. The effect of formins on tubulin acetylation is the combined result of their ability to activate α-TAT1 gene transcription and of their capacity to regulate microtubule stabilization. The contribution of these two mechanisms in different formins is discussed, particularly with respect to INF2, a formin that is mutated in hereditary human renal and neurodegenerative disorders.
Keywords: Actin homeostasis; Formins; INF2; Microtubules; Myocardin-related transcription factor; Serum response factor; Tubulin acetylation; α-Tubulin acetyltransferase 1.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

