A pharmacodynamic study of sirolimus and metformin in patients with advanced solid tumors
- PMID: 29948021
- PMCID: PMC6739841
- DOI: 10.1007/s00280-018-3619-3
A pharmacodynamic study of sirolimus and metformin in patients with advanced solid tumors
Abstract
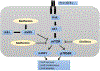
Background: Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor. Metformin may potentiate mTOR inhibition by sirolimus while mitigating its adverse effects. We conducted a pilot study to test this hypothesis.
Methods: Patients with advanced solid tumor were treated with sirolimus for 7 days followed by randomization to either sirolimus with metformin (Arm A) or sirolimus (Arm B) until day 21. From day 22 onwards, all patients received sirolimus and metformin. The primary aim was to compare the change in phospho-p70S6K (pp70S6K) in peripheral blood mononuclear cells (PBMC) from day 8 to day 22 using a two-sample t test. Secondary aims were objective response rate, toxicity, and other serum pharmacodynamic biomarkers (e.g., fasting glucose, triglycerides, insulin, C-peptide, IGF-1, IGF-1R, IGF-BP, and leptin).
Results: 24 patients were enrolled, with 18 evaluable for the primary endpoint. There was no significant difference in mean change in pp70S6K in arm A vs. arm B (- 0.12 vs. - 0.16; P = 0.64). Similarly, there were no significant differences in other serum pharmacodynamic biomarkers. There were no partial responses. There were no dose-limiting or unexpected toxicities.
Conclusions: Adding metformin to sirolimus, although well tolerated, was not associated with significant changes in pp70S6K in PBMC or other serum pharmacodynamic biomarkers.
Impact: Combining metformin with sirolimus did not improve mTOR inhibition.
Keywords: Metformin; P70S6K and mTOR; Pharmacodynamics; Sirolimus; Solid tumors.
Conflict of interest statement
Figures
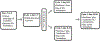


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

