Outcome of Patients Treated Within and Outside a Randomized Clinical Trial on Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: Extrapolation of a Randomized Clinical Trial (CROSS)
- PMID: 29948420
- PMCID: PMC6029046
- DOI: 10.1245/s10434-018-6554-y
Outcome of Patients Treated Within and Outside a Randomized Clinical Trial on Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: Extrapolation of a Randomized Clinical Trial (CROSS)
Abstract
Background: Randomized clinical trials (RCTs) can provide a high level of evidence for medical decision making, but it is unclear if the results apply to patients treated outside such trials.
Objective: The aim of this study was to retrospectively compare outcomes of patients with esophageal cancer treated within and outside an RCT.
Methods: All patients receiving neoadjuvant chemoradiotherapy (nCRT) plus surgery for esophageal cancer between 2002 and 2008 (ChemoRadiotherapy for Esophageal cancer followed by Surgery Study [CROSS] cohort) who participated in multicenter, phase II-III trials were compared with patients who underwent the same treatment outside the trial between 2008 and 2013 (post-CROSS cohort). The differences between these cohorts were analyzed using t tests, while logistic regression models were used to evaluate adverse events. Overall and disease-free survival were calculated using the Kaplan-Meier method and Cox regression analyses.
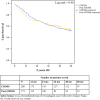
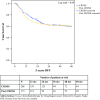
Results: A total of 208 CROSS patients and 173 post-CROSS patients were included in this study. Patients from the post-CROSS cohort were older, had more co morbidities, and had poorer performance status. Clinical N stage, but not cT stage, was worse in the post-CROSS cohort. There were no statistically significant differences in adverse events (pulmonary, cardiac, or anastomotic complications) or survival between the comparison cohorts.
Conclusion: The outcomes of patients treated with nCRT plus esophagectomy for cancer have a high external consistency and can be extrapolated to the daily practice of physicians involved in the treatment and care of esophageal cancer patients.
Conflict of interest statement
There are no financial interests or potential conflicts of interest.
Figures
Similar articles
-
Analysis of patients scheduled for neoadjuvant therapy followed by surgery for esophageal cancer, who never made it to esophagectomy.World J Surg Oncol. 2019 May 27;17(1):89. doi: 10.1186/s12957-019-1630-8. World J Surg Oncol. 2019. PMID: 31133018 Free PMC article.
-
Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome?Ann Surg Oncol. 2013 Dec;20(13):4245-51. doi: 10.1245/s10434-013-3139-7. Ann Surg Oncol. 2013. PMID: 23959050
-
Neoadjuvant Chemoradiation Versus Upfront Esophagectomy in Clinical Stage II and III Esophageal Squamous Cell Carcinoma.Ann Surg Oncol. 2019 Feb;26(2):506-513. doi: 10.1245/s10434-018-7060-y. Epub 2018 Nov 14. Ann Surg Oncol. 2019. PMID: 30430325
-
Current Advancement in Multidisciplinary Treatment for Resectable cStage II/III Esophageal Squamous Cell Carcinoma in Japan.Ann Thorac Cardiovasc Surg. 2016 Oct 20;22(5):275-283. doi: 10.5761/atcs.ra.16-00111. Epub 2016 Jul 6. Ann Thorac Cardiovasc Surg. 2016. PMID: 27384595 Free PMC article. Review.
-
Impact of Neoadjuvant Chemoradiation on Short-Term Outcomes for Esophageal Squamous Cell Carcinoma Patients: A Meta-analysis.Ann Surg Oncol. 2016 Oct;23(11):3632-3640. doi: 10.1245/s10434-016-5298-9. Epub 2016 Jun 8. Ann Surg Oncol. 2016. PMID: 27278203 Review.
Cited by
-
Long-term effects of radiation prior to surgery and chemotherapy on survival of esophageal cancer undergoing surgery.Medicine (Baltimore). 2019 Oct;98(43):e17617. doi: 10.1097/MD.0000000000017617. Medicine (Baltimore). 2019. PMID: 31651875 Free PMC article.
-
Which test for crossing survival curves? A user's guideline.BMC Med Res Methodol. 2022 Jan 30;22(1):34. doi: 10.1186/s12874-022-01520-0. BMC Med Res Methodol. 2022. PMID: 35094686 Free PMC article. Review.
-
Tumor Lymphocyte Infiltration Is Correlated with a Favorable Tumor Regression Grade after Neoadjuvant Treatment for Esophageal Adenocarcinoma.J Pers Med. 2022 Apr 13;12(4):627. doi: 10.3390/jpm12040627. J Pers Med. 2022. PMID: 35455743 Free PMC article.
-
Effect of Extending the Original CROSS Criteria on Tumor Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer Patients: A National Multicenter Cohort Analysis.Ann Surg Oncol. 2021 Jul;28(7):3951-3960. doi: 10.1245/s10434-020-09372-y. Epub 2020 Nov 28. Ann Surg Oncol. 2021. PMID: 33249520 Free PMC article.
-
Neoadjuvant Therapy for Locally Advanced Esophageal Cancers.Front Oncol. 2022 Apr 7;12:734581. doi: 10.3389/fonc.2022.734581. eCollection 2022. Front Oncol. 2022. PMID: 35463306 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

