SNAPshots of the MCHR1: a Comparison Between the PET-Tracers [18F]FE@SNAP and [11C]SNAP-7941
- PMID: 29948643
- PMCID: PMC6449294
- DOI: 10.1007/s11307-018-1212-0
SNAPshots of the MCHR1: a Comparison Between the PET-Tracers [18F]FE@SNAP and [11C]SNAP-7941
Abstract
Purpose: The melanin-concentrating hormone receptor 1 (MCHR1) has become an important pharmacological target, since it may be involved in various diseases, such as diabetes, insulin resistance, and obesity. Hence, a suitable positron emission tomography radiotracer for the in vivo assessment of the MCHR1 pharmacology is imperative. The current paper contrasts the extensive in vitro, in vivo, and ex vivo assessments of the radiotracers [18F]FE@SNAP and [11C]SNAP-7941 and provides comprehensive information about their biological and physicochemical properties. Furthermore, it examines their suitability for first-in-man imaging studies.
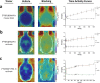
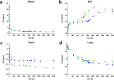
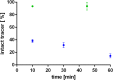
Procedures: Kinetic real-time cell-binding studies with [18F]FE@SNAP and [11C]SNAP-7941 were conducted on adherent Chines hamster ovary (CHO-K1) cells stably expressing the human MCHR1 and MCHR2. Small animal imaging studies on mice and rats were performed under displacement and baseline conditions, as well as after pretreatment with the P-glycoprotein/breast cancer resistant protein inhibitor tariquidar. After the imaging studies, detailed analyses of the ex vivo biodistribution were performed. Ex vivo metabolism was determined in rat blood and brain and analyzed at various time points using a quantitative radio-HPLC assay.
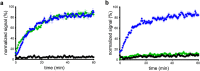
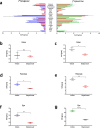
Results: [11C]SNAP-7941 demonstrates high uptake on CHO-K1-hMCHR1 cells, whereas no uptake was detected for the CHO-K1-hMCHR2 cells. In contrast, [18F]FE@SNAP evinced binding to CHO-K1-hMCHR1 and CHO-K1-hMCHR2 cells. Imaging studies with [18F]FE@SNAP and [11C]SNAP-7941 showed an increased brain uptake after tariquidar pretreatment in mice, as well as in rats, and exhibited a significant difference between the time-activity curves of the baseline and blocking groups. Biodistribution of both tracers demonstrated a decreased uptake after displacement. [11C]SNAP-7941 revealed a high metabolic stability in rats, whereas [18F]FE@SNAP was rapidly metabolized.
Conclusions: Both radiotracers demonstrate appropriate imaging properties for the MCHR1. However, the pronounced metabolic stability as well as superior selectivity and affinity of [11C]SNAP-7941 underlines the decisive superiority over [18F]FE@SNAP.
Keywords: Ex vivo; In vitro; In vivo; MCHR1; PET; Small animal imaging; [11C]SNAP-7941; [18F]FE@SNAP.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

