An historical overview over Pharmacovigilance
- PMID: 29948743
- PMCID: PMC6132952
- DOI: 10.1007/s11096-018-0657-1
An historical overview over Pharmacovigilance
Abstract
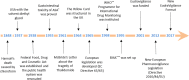
Pharmacovigilance started about 170 years ago, although it was not yet named as such at that time. It is structured activity in the professional health field, with important social and commercial implications aimed at monitoring the risk/benefit ratio of drugs, improving patient's safety and the quality of life. In this commentary we report the milestones of pharmacovigilance up to the present day, in order to understand all the steps that have characterized the historical evolution; from the first reports, which were essentially letters or warnings sent by clinicians to publishers of important and famous scientific journals, up to today's modern and ultra-structured electronic registries. The historical phases also help us to understand why pharmacovigilance helped us to achieve such important results for man's health and for pharmacology itself, and to identify the challenges that await Pharmacovigilance in future years.
Keywords: Adverse drug reactions; History; Legislation; Pharmacovigilances; Signal detection; Thalidomide.
Conflict of interest statement
None.
Figures
References
-
- Pharmacovigilance Risk Assessment Committee: PRAC strategy on measuring the impact of Pharmacovigilance activities. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/01/WC500....
-
- Commission on Anaesthetics. Lancet. 1893; i:629–38.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical