Conducting a Time Trade-Off Study Alongside a Clinical Trial: A Case Study and Recommendations
- PMID: 29949064
- PMCID: PMC6393276
- DOI: 10.1007/s41669-018-0084-1
Conducting a Time Trade-Off Study Alongside a Clinical Trial: A Case Study and Recommendations
Erratum in
-
Correction to: Conducting a Time Trade-Off Study Alongside a Clinical Trial: A Case Study and Recommendations.Pharmacoecon Open. 2019 Sep;3(3):427. doi: 10.1007/s41669-019-0145-0. Pharmacoecon Open. 2019. PMID: 31123930 Free PMC article.
Abstract
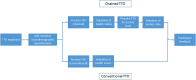
Time trade-off (TTO) is an established method in health economics to elicit and value individuals' preferences for different health states. These preferences are expressed in the form of health-state utilities that are typically used to measure health-related quality of life and calculate quality-adjusted life-years in an economic evaluation. The TTO approach to directly elicit health-state utilities is particularly valuable when generic instruments (e.g. EQ-5D) may not fully capture changes in utility in a clinical trial. However, there is limited guidance on how a TTO study should be conducted alongside a clinical trial despite it being a valuable tool. We present an account of the design and development of a TTO study within a clinical trial as a case study. We describe the development of materials needed for the TTO interviews, the piloting of the TTO materials and interview process, and recommendations for future TTO studies. This paper provides a practical guide and reference for future applications of the TTO method alongside a clinical trial.
Conflict of interest statement
Jing Shen, Sarah Hill, David Mott, Matthew Breckons, Luke Vale and Rob Pickard have no conflicts of interest.
Figures
References
-
- National Institute for Clinical Excellence. Guide to the methods of technology appraisal. London: NICE. 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword. - PubMed
-
- van Reenen M, Oppe M. EQ-5D-3L User Guide: basic information on how to use the EQ-5D-3L instrument Rotterdam: EuroQol Group; 2015. Available from: https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-3L_UserGuide_2015.pdf.
-
- van Reenen M, Janssen B. EQ-5D-5L user guide: basic information on how to use the EQ-5D-5L instrument Rotterdam: EuroQol Group; 2015. Available from: https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources