Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection
- PMID: 29949404
- PMCID: PMC6171039
- DOI: 10.1152/ajpcell.00095.2018
Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection
Abstract
The blood-brain barrier (BBB) is a physical and biochemical barrier that precisely controls cerebral homeostasis. It also plays a central role in the regulation of blood-to-brain flux of endogenous and exogenous xenobiotics and associated metabolites. This is accomplished by molecular characteristics of brain microvessel endothelial cells such as tight junction protein complexes and functional expression of influx and efflux transporters. One of the pathophysiological features of ischemic stroke is disruption of the BBB, which significantly contributes to development of brain injury and subsequent neurological impairment. Biochemical characteristics of BBB damage include decreased expression and altered organization of tight junction constituent proteins as well as modulation of functional expression of endogenous BBB transporters. Therefore, there is a critical need for development of novel therapeutic strategies that can protect against BBB dysfunction (i.e., vascular protection) in the setting of ischemic stroke. Such strategies include targeting tight junctions to ensure that they maintain their correct structure or targeting transporters to control flux of physiological substrates for protection of endothelial homeostasis. In this review, we will describe the pathophysiological mechanisms in cerebral microvascular endothelial cells that lead to BBB dysfunction following onset of stroke. Additionally, we will utilize this state-of-the-art knowledge to provide insights on novel pharmacological strategies that can be developed to confer BBB protection in the setting of ischemic stroke.
Keywords: blood-brain barrier; endothelial cell; oxidative stress; tight junctions; transporters.
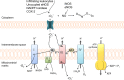
Figures





References
-
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci 91: 2525–2538, 2002. doi: 10.1002/jps.10256. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

