Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer
- PMID: 29949494
- PMCID: PMC8288034
- DOI: 10.1056/NEJMoa1800536
Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer
Abstract
Background: Men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising prostate-specific antigen (PSA) level are at high risk for metastasis. We hypothesized that enzalutamide, which prolongs overall survival among patients with metastatic, castration-resistant prostate cancer, would delay metastasis in men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising PSA level.
Methods: In this double-blind, phase 3 trial, we randomly assigned, in a 2:1 ratio, men with nonmetastatic, castration-resistant prostate cancer and a PSA doubling time of 10 months or less who were continuing androgen-deprivation therapy to receive enzalutamide (at a dose of 160 mg) or placebo once daily. The primary end point was metastasis-free survival (defined as the time from randomization to radiographic progression or as the time to death without radiographic progression).
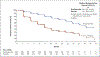
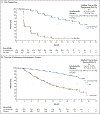
Results: A total of 1401 patients (median PSA doubling time, 3.7 months) underwent randomization. As of June 28, 2017, a total of 219 of 933 patients (23%) in the enzalutamide group had metastasis or had died, as compared with 228 of 468 (49%) in the placebo group. The median metastasis-free survival was 36.6 months in the enzalutamide group versus 14.7 months in the placebo group (hazard ratio for metastasis or death, 0.29; 95% confidence interval, 0.24 to 0.35; P<0.001). The time to the first use of a subsequent antineoplastic therapy was longer with enzalutamide treatment than with placebo (39.6 vs. 17.7 months; hazard ratio, 0.21; P<0.001; such therapy was used in 15% vs. 48% of patients) as was the time to PSA progression (37.2 vs. 3.9 months; hazard ratio, 0.07; P<0.001; progression occurred in 22% vs. 69% of patients). At the first interim analysis of overall survival, 103 patients (11%) receiving enzalutamide and 62 (13%) receiving placebo had died. Adverse events of grade 3 or higher occurred in 31% of the patients receiving enzalutamide, as compared with 23% of those receiving placebo.
Conclusions: Among men with nonmetastatic, castration-resistant prostate cancer with a rapidly rising PSA level, enzalutamide treatment led to a clinically meaningful and significant 71% lower risk of metastasis or death than placebo. Adverse events were consistent with the established safety profile of enzalutamide. (Funded by Pfizer and Astellas Pharma; PROSPER ClinicalTrials.gov number, NCT02003924 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Progress in Nonmetastatic Prostate Cancer.N Engl J Med. 2018 Jun 28;378(26):2531-2532. doi: 10.1056/NEJMe1805733. N Engl J Med. 2018. PMID: 29949488 No abstract available.
-
Re: Enzalutamide in Men with Nonmetastatic, Castration-resistant Prostate Cancer.Eur Urol. 2018 Dec;74(6):845. doi: 10.1016/j.eururo.2018.08.002. Epub 2018 Sep 1. Eur Urol. 2018. PMID: 30177288 No abstract available.
-
Enzalutamide in Castration-Resistant Prostate Cancer.N Engl J Med. 2018 Oct 4;379(14):1380. doi: 10.1056/NEJMc1810065. N Engl J Med. 2018. PMID: 30284800 No abstract available.
-
Enzalutamide in Castration-Resistant Prostate Cancer.N Engl J Med. 2018 Oct 4;379(14):1380-1. doi: 10.1056/NEJMc1810065. N Engl J Med. 2018. PMID: 30284801 No abstract available.
-
New drug option for men with aggressive prostate cancer.Cancer. 2018 Oct 1;124(19):3797. doi: 10.1002/cncr.31753. Cancer. 2018. PMID: 30412304 No abstract available.
-
Re: Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer.J Urol. 2019 Jan;201(1):31. doi: 10.1097/01.ju.0000550155.71767.b1. J Urol. 2019. PMID: 30577375 No abstract available.
-
Challenges of treatment selection in the older prostate patient.J Geriatr Oncol. 2019 Jul;10(4):666-668. doi: 10.1016/j.jgo.2019.01.009. Epub 2019 Jan 20. J Geriatr Oncol. 2019. PMID: 30674419 No abstract available.
-
Kastrationsresistentes Prostatakarzinom: Enzalutamid besser als Placebo.Aktuelle Urol. 2019 Feb;50(1):12-14. doi: 10.1055/a-0830-9049. Epub 2019 Feb 7. Aktuelle Urol. 2019. PMID: 30731496 German. No abstract available.
-
Metastases-free survival rate in non-metastatic castration-resistant prostate cancer: Now living on its own!Actas Urol Esp (Engl Ed). 2019 May;43(4):167-168. doi: 10.1016/j.acuro.2019.01.002. Epub 2019 Mar 4. Actas Urol Esp (Engl Ed). 2019. PMID: 30846288 English, Spanish. No abstract available.
References
-
- Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate non-metastatic prostate cancer. J Clin Oncol 2005; 23: 2918–25. - PubMed
-
- Afshar M, Evison F, James ND, Patel P. Shifting paradigms in the estimation of survival for castration-resistant prostate cancer: a tertiary academic center experience. Urol Oncol 2015; 33(8): 338.e1–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
