Patient reported measures of informed consent for clinical trials: A systematic review
- PMID: 29949627
- PMCID: PMC6021104
- DOI: 10.1371/journal.pone.0199775
Patient reported measures of informed consent for clinical trials: A systematic review
Abstract
Introduction: The subjective assessment of the adequacy of informed consent for clinical trials, and the potential difficulties associated with it, has led several studies to develop objective measures of informed consent for clinical trials. These objective measures of informed consent are often specific to a particular population or clinical condition and largely focus on understanding of (some or all of) the key elements of informed consent. Many of the developed tools are study-specific, but some validated measures exist. Of these validated measures, those which are reported by participants are of particular interest. Whether these objective tools conceptualize and measure informed consent in the same way is not known. As such, it is not clear whether meta-analyzing data from studies reporting different tools is worthwhile. The aim of this systematic review was to critically appraise the evidence on the overall conceptualisation and item content of validated patient reported measures of informed consent for clinical trials, and to identify core domains of potential importance for informed consent.
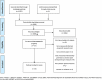
Methods: A systematic search of the literature was conducted to identify relevant articles that described the development, and/or validation, of patient-reported measures of adequacy of informed consent for randomised controlled trials. Data was synthesised by classifying the items identified into domains and sub-domains which were determined by the nomenclature reported in included studies. Both for descriptions of included studies and of the instruments reported in those studies, descriptive statistics were used to describe general information and instrument detail. A narrative synthesis of the instruments and their inter-related domains and subdomains was conducted to identify areas of both convergence and divergence.
Results: The search identified 8193 citations. After screening titles and abstracts, 29 full text articles were retrieved for further assessment. Of these 29, 14 complied with our pre-specified inclusion criteria with 15 not being eligible. Of the 14 instruments, three explicitly reported a theoretical or conceptual framework underpinning their development, a further three implicitly referred to the 'conceptual dimensions of informed consent' or 'principles of research ethics' as informing their development and eight reported no guiding theoretical framework. Only three of the 14 studies reported patient or public involvement in the development of the tool. One hundred and seventy nine items were included across the 14 instruments. The primary focus of the instruments was on understanding. Five core domains were identified which included: Autonomy; Consequences; Expectations; Purpose; and Individualisation. There was substantial variability in the coverage of different domains across measures.
Conclusions: This study demonstrated the variability in the theoretical underpinning, development and domain coverage of existing patient-reported measures of informed consent for clinical trials. The conceptualisation of informed consent could benefit from being extended from a narrow focus on understanding to include broader considerations of decision-making. Meaningful involvement of potential trial participants during development of measures critical for tool relevance is also lacking. The identification of the key domains relevant to all stakeholders which could be measured to assess the informed consent process for clinical trials is needed.
Conflict of interest statement
The authors have declared that no competing interests exist. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures
References
-
- World Medical Association (WMA): WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects Ferney-Voltaire: WMA; 2008. http://www.wma.net/en/30publications/10policies/b3/index.html
-
- International Conference on Harmonisation (ICH): ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1) Geneva: ICH; 1996. [Step 4 version]
-
- Beauchamp TL & Childress JF. Principles of Biomedical Ethics 2012. Oxford: Oxford University Press, 7.
-
- REGULATION (EU) No 536/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC.
-
- Kinnersley P, Phillips K, Savage K, Kelly MJ, Farrell E, Morgan B, et al.Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst Rev. 2013. July 6;(7):CD009445 doi: 10.1002/14651858.CD009445.pub2 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials