Development of a high-throughput assay to detect antibody inhibition of low pH induced conformational changes of influenza virus hemagglutinin
- PMID: 29949635
- PMCID: PMC6021090
- DOI: 10.1371/journal.pone.0199683
Development of a high-throughput assay to detect antibody inhibition of low pH induced conformational changes of influenza virus hemagglutinin
Abstract
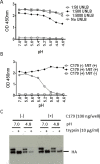
Many broadly neutralizing antibodies (bnAbs) bind to conserved areas of the hemagglutinin (HA) stalk region and can inhibit the low pH induced HA conformational changes necessary for viral membrane fusion activity. We developed and evaluated a high-throughput virus-free and cell-free ELISA based low pH induced HA Conformational Change Inhibition Antibody Detection Assay (HCCIA) and a complementary proteinase susceptibility assay. Human serum samples (n = 150) were tested by HCCIA using H3 recombinant HA. Optical density (OD) ratios of mAb HC31 at pH 4.8 to pH 7.0 ranged from 0.87 to 0.09. Our results demonstrated that low pH induced HA conformational change inhibition antibodies (CCI) neutralized multiple H3 strains after removal of head-binding antibodies. The results suggest that HCCIA can be utilized to detect and characterize CCI in sera, that are potentially broadly neutralizing, and serves as a useful tool for evaluating universal vaccine candidates targeting the HA stalk.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses.J Virol. 2014 Jul;88(13):7130-44. doi: 10.1128/JVI.00420-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719430 Free PMC article.
-
Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth.J Virol. 2020 Feb 28;94(6):e01035-19. doi: 10.1128/JVI.01035-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31826999 Free PMC article.
-
Conformational Stability of the Hemagglutinin of H5N1 Influenza A Viruses Influences Susceptibility to Broadly Neutralizing Stem Antibodies.J Virol. 2018 May 29;92(12):e00247-18. doi: 10.1128/JVI.00247-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593038 Free PMC article.
-
A broadly neutralizing human monoclonal antibody directed against a novel conserved epitope on the influenza virus H3 hemagglutinin globular head.J Virol. 2014 Jun;88(12):6743-50. doi: 10.1128/JVI.03562-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696468 Free PMC article.
-
Influenza Hemagglutinin Structures and Antibody Recognition.Cold Spring Harb Perspect Med. 2020 Aug 3;10(8):a038778. doi: 10.1101/cshperspect.a038778. Cold Spring Harb Perspect Med. 2020. PMID: 31871236 Free PMC article. Review.
Cited by
-
Influenza Virus Entry inhibitors.Adv Exp Med Biol. 2022;1366:123-135. doi: 10.1007/978-981-16-8702-0_8. Adv Exp Med Biol. 2022. PMID: 35412138 Review.
-
A conserved histidine in Group-1 influenza subtype hemagglutinin proteins is essential for membrane fusion activity.Virology. 2019 Oct;536:78-90. doi: 10.1016/j.virol.2019.08.005. Epub 2019 Aug 6. Virology. 2019. PMID: 31401467 Free PMC article.
-
Antibody Landscape Analysis following Influenza Vaccination and Natural Infection in Humans with a High-Throughput Multiplex Influenza Antibody Detection Assay.mBio. 2021 Feb 2;12(1):e02808-20. doi: 10.1128/mBio.02808-20. mBio. 2021. PMID: 33531397 Free PMC article.
-
Identification of novel influenza A virus exposures by an improved high-throughput multiplex MAGPIX platform and serum adsorption.Influenza Other Respir Viruses. 2020 Mar;14(2):129-141. doi: 10.1111/irv.12695. Epub 2019 Nov 8. Influenza Other Respir Viruses. 2020. PMID: 31701647 Free PMC article.
References
-
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9(10):e1003657 doi: 10.1371/journal.ppat.1003657 - DOI - PMC - PubMed
-
- Wu NC, Wilson IA. A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus. Journal of molecular biology. 2017;429(17):2694–709. doi: 10.1016/j.jmb.2017.06.015 - DOI - PMC - PubMed
-
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9(6):669–83. doi: 10.1586/eri.11.51 - DOI - PubMed
-
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14(3):167–82. doi: 10.1038/nrd4529 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

