Mitoregulin: A lncRNA-Encoded Microprotein that Supports Mitochondrial Supercomplexes and Respiratory Efficiency
- PMID: 29949756
- PMCID: PMC6091870
- DOI: 10.1016/j.celrep.2018.06.002
Mitoregulin: A lncRNA-Encoded Microprotein that Supports Mitochondrial Supercomplexes and Respiratory Efficiency
Abstract
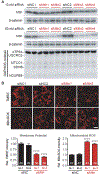
Mitochondria are composed of many small proteins that control protein synthesis, complex assembly, metabolism, and ion and reactive oxygen species (ROS) handling. We show that a skeletal muscle- and heart-enriched long non-coding RNA, LINC00116, encodes a highly conserved 56-amino-acid microprotein that we named mitoregulin (Mtln). Mtln localizes to the inner mitochondrial membrane, where it binds cardiolipin and influences protein complex assembly. In cultured cells, Mtln overexpression increases mitochondrial membrane potential, respiration rates, and Ca2+ retention capacity while decreasing mitochondrial ROS and matrix-free Ca2+. Mtln-knockout mice display perturbations in mitochondrial respiratory (super)complex formation and activity, fatty acid oxidation, tricarboxylic acid (TCA) cycle enzymes, and Ca2+ retention capacity. Blue-native gel electrophoresis revealed that Mtln co-migrates alongside several complexes, including the complex I assembly module, complex V, and supercomplexes. Under denaturing conditions, Mtln remains in high-molecular-weight complexes, supporting its role as a sticky molecular tether that enhances respiratory efficiency by bolstering protein complex assembly and/or stability.
Keywords: ROS; assembly factor; fatty acid oxidation; micropeptide; microprotein; mitochondria; mitochondrial calcium; respirasome; sORF; supercomplexes.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




References
-
- Anderson EJ, and Neufer PD (2006). Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am. J. Physiol. Cell Physiol 290, C844–C851. - PubMed
-
- Anderson RD, Haskell RE, Xia H, Roessler BJ, and Davidson BL (2000). A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7, 1034–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

