NMDA Receptor Autoantibodies in Autoimmune Encephalitis Cause a Subunit-Specific Nanoscale Redistribution of NMDA Receptors
- PMID: 29949761
- PMCID: PMC6060408
- DOI: 10.1016/j.celrep.2018.05.096
NMDA Receptor Autoantibodies in Autoimmune Encephalitis Cause a Subunit-Specific Nanoscale Redistribution of NMDA Receptors
Abstract
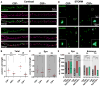
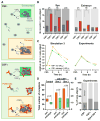
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a severe neuropsychiatric disorder mediated by autoantibodies against the GluN1 subunit of the NMDAR. Patients' antibodies cause cross-linking and internalization of NMDAR, but the synaptic events leading to depletion of NMDAR are poorly understood. Using super-resolution microscopy, we studied the effects of the autoantibodies on the nanoscale distribution of NMDAR in cultured neurons. Our findings show that, under control conditions, NMDARs form nanosized objects and patients' antibodies increase the clustering of synaptic and extrasynaptic receptors inside the nano-objects. This clustering is subunit specific and predominantly affects GluN2B-NMDARs. Following internalization, the remaining surface NMDARs return to control clustering levels but are preferentially retained at the synapse. Monte Carlo simulations using a model in which antibodies induce NMDAR cross-linking and disruption of interactions with other proteins recapitulated these results. Finally, activation of EphB2 receptor partially antagonized the antibody-mediated disorganization of the nanoscale surface distribution of NMDARs.
Keywords: GluN2 subunits; NMDAR encephalitis; STORM; antibody; autoimmune; pathogenic mechanism; super-resolution microscopy; synaptic proteins.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
J.D. holds a patent for the use of Ma2 and NMDAR as an autoantibody test, receives royalties related to this test, and has an unrestricted research grant from Euroimmun.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

