Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae)
- PMID: 29949847
- PMCID: PMC6099557
- DOI: 10.3390/molecules23071532
Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae)
Abstract
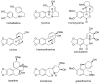
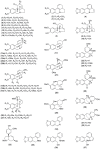
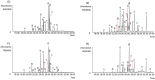
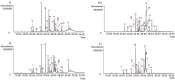
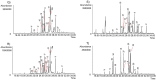
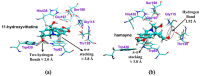
Amaryllidaceae plants are the commercial source of galanthamine, an alkaloid approved for the clinical treatment of Alzheimer’s disease. The chemistry and bioactivity of Chilean representatives of Rhodophiala genus from the family of Amaryllidaceae have not been widely studied so far. Ten collections of five different Chilean Rhodophiala were analyzed in vitro for activity against enzymes such as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) as well as for their alkaloid composition by GC-MS. To obtain an insight into the potential AChE and BuChE inhibitory activity of the alkaloids identified in the most active samples, docking experiments were carried out. Although galanthamine was found neither in aerial parts nor in bulbs of R. splendens, these plant materials were the most active inhibitors of AChE (IC50: 5.78 and 3.62 μg/mL, respectively) and BuChE (IC50: 16.26 and 14.37 μg/mL, respectively). Some 37 known alkaloids and 40 still unidentified compounds were detected in the samples, suggesting high potential in the Chilean Amaryllidaceae plants as sources of both novel bioactive agents and new alkaloids.
Keywords: AChE; BuChE; GC-MS; Rhodophiala; alkaloids; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




Similar articles
-
Clinanthus microstephium, an Amaryllidaceae Species with Cholinesterase Inhibitor Alkaloids: Structure-Activity Analysis of Haemanthamine Skeleton Derivatives.Chem Biodivers. 2019 May;16(5):e1800662. doi: 10.1002/cbdv.201800662. Epub 2019 Apr 9. Chem Biodivers. 2019. PMID: 30801949
-
Hippeastrum reticulatum (Amaryllidaceae): Alkaloid Profiling, Biological Activities and Molecular Docking.Molecules. 2017 Dec 9;22(12):2191. doi: 10.3390/molecules22122191. Molecules. 2017. PMID: 29232852 Free PMC article.
-
Alkaloids of Amaryllidaceae as Inhibitors of Cholinesterases (AChEs and BChEs): An Integrated Bioguided Study.Phytochem Anal. 2018 Mar;29(2):217-227. doi: 10.1002/pca.2736. Epub 2017 Oct 17. Phytochem Anal. 2018. PMID: 29044771
-
The Biological Activity of Alkaloids from the Amaryllidaceae: From Cholinesterases Inhibition to Anticancer Activity.Nat Prod Commun. 2016 Oct;11(10):1587-1594. Nat Prod Commun. 2016. PMID: 30549626 Review.
-
Pharmacological and toxicological effects of Amaryllidaceae.Braz J Biol. 2023 Dec 15;83:e277092. doi: 10.1590/1519-6984.277092. eCollection 2023. Braz J Biol. 2023. PMID: 38126586 Review.
Cited by
-
Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities.Metabolites. 2020 Jul 28;10(8):309. doi: 10.3390/metabo10080309. Metabolites. 2020. PMID: 32731456 Free PMC article.
-
Alkaloid Profiling, Anti-Enzymatic and Antiproliferative Activity of the Endemic Chilean Amaryllidaceae Phycella cyrtanthoides.Metabolites. 2022 Feb 18;12(2):188. doi: 10.3390/metabo12020188. Metabolites. 2022. PMID: 35208261 Free PMC article.
-
Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability.Antioxidants (Basel). 2021 Jul 30;10(8):1230. doi: 10.3390/antiox10081230. Antioxidants (Basel). 2021. PMID: 34439478 Free PMC article.
-
Phytochemical Characterization and In Vitro and In Silico Biological Studies from Ferns of Genus Blechnum (Blechnaceae, Polypodiales).Antioxidants (Basel). 2023 Feb 21;12(3):540. doi: 10.3390/antiox12030540. Antioxidants (Basel). 2023. PMID: 36978788 Free PMC article.
-
In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile.Plants (Basel). 2024 Sep 30;13(19):2756. doi: 10.3390/plants13192756. Plants (Basel). 2024. PMID: 39409626 Free PMC article.
References
-
- Bastida J., Berkov S., Torras L., Pigni N.B., de Andrade J.P., Martínez V., Codina C., Viladomat F. Chemical and biological aspects of Amaryllidaceae alkaloids. In: Muñoz-Torrero D., editor. Recent Advances in Pharmaceutical Sciences. Transworld Research Network; Kerala, India: 2011. pp. 65–100.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

