Properties and Stability of Perilla Seed Protein-Stabilized Oil-in-Water Emulsions: Influence of Protein Concentration, pH, NaCl Concentration and Thermal Treatment
- PMID: 29949852
- PMCID: PMC6100609
- DOI: 10.3390/molecules23071533
Properties and Stability of Perilla Seed Protein-Stabilized Oil-in-Water Emulsions: Influence of Protein Concentration, pH, NaCl Concentration and Thermal Treatment
Abstract
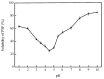
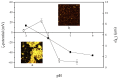
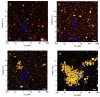
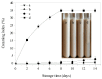
Perilla seed protein (PSP) was extracted from defatted perilla seed meal and applied in oil-in-water (O/W) emulsions as an emulsifier. We investigated the influences of protein concentration (0.25⁻1.5 wt %), pH (3.0⁻9.0), NaCl concentration (0⁻350 mmol/L) and thermal treatment (70⁻90 °C, 30 min) on the physical characteristics of O/W emulsions, including volume-average diameter, ζ-potential, interfacial protein concentration, microstructure and so on. Results showed that increasing PSP concentration would decrease the d4,3 and a 1.0 wt % PSP concentration was sufficient to ensure the stability of emulsion. Under pH 3.0⁻9.0, emulsions were stable except at pH 3.0⁻5.0 which was proximal to the isoelectric point (pH 4.5) of PSP. At high NaCl concentrations (250⁻350 mmol/L), the emulsions exhibited relatively lower absolute ζ-potential values and a large number of aggregated droplets. A moderate thermal treatment temperature (e.g., 70 °C) was favorable for the emulsion against aggregation and creaming. However, when 90 °C thermal treatment was performed, a clear layer separation was observed after 2 weeks storage and the emulsion showed a poor stability. The findings of this work are of great importance for the utilization and development of PSP as an emulsifier for food emulsions.
Keywords: NaCl concentration; O/W emulsion; pH; perilla seed protein; thermal treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Longvah T., Deosthale Y.G. Effect of dehulling, cooking and roasting on the protein quality of Perilla frutescens seed. Food Chem. 1998;63:519–523. doi: 10.1016/S0308-8146(98)00030-2. - DOI
-
- Zhu J., Fu Q. Optimization of ultrasound-assisted extraction process of perilla seed meal proteins. Food Sci. Biotechnol. 2012;21:1701–1706. doi: 10.1007/s10068-012-0226-7. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

