The Desmosomal Cadherin Desmoglein-2 Experiences Mechanical Tension as Demonstrated by a FRET-Based Tension Biosensor Expressed in Living Cells
- PMID: 29949915
- PMCID: PMC6070948
- DOI: 10.3390/cells7070066
The Desmosomal Cadherin Desmoglein-2 Experiences Mechanical Tension as Demonstrated by a FRET-Based Tension Biosensor Expressed in Living Cells
Abstract
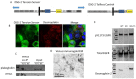
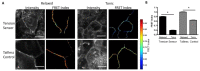
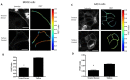
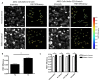
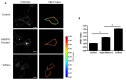
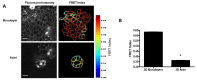
Cell-cell junctions are critical structures in a number of tissues for mechanically coupling cells together, cell-to-cell signaling, and establishing a barrier. In many tissues, desmosomes are an important component of cell-cell junctions. Loss or impairment of desmosomes presents with clinical phenotypes in the heart and skin as cardiac arrhythmias and skin blistering, respectively. Because heart and skin are tissues that are subject to large mechanical stresses, we hypothesized that desmosomes, similar to adherens junctions, would also experience significant tensile loading. To directly measure mechanical forces across desmosomes, we developed and validated a desmoglein-2 (DSG-2) force sensor, using the existing TSmod Förster resonance energy transfer (FRET) force biosensor. When expressed in human cardiomyocytes, the force sensor reported high tensile loading of DSG-2 during contraction. Additionally, when expressed in Madin-Darby canine kidney (MDCK) epithelial or epidermal (A431) monolayers, the sensor also reported tensile loading. Finally, we observed higher DSG-2 forces in 3D MDCK acini when compared to 2D monolayers. Taken together, our results show that desmosomes experience low levels of mechanical tension in resting cells, with significantly higher forces during active loading.
Keywords: cell biophysics; desmosomes; mechanobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Borghi N., Sorokina M., Shcherbakova O.G., Weis W.I., Pruitt B.L., Nelson W.J., Dunn A.R. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. USA. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

