Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function
- PMID: 29949922
- PMCID: PMC6071034
- DOI: 10.3390/cells7070067
Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function
Abstract
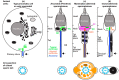
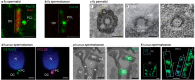
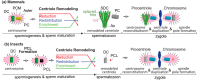
Centrioles are ancient subcellular protein-based organelles that maintain a conserved number and structure across many groups of eukaryotes. Centriole number (two per cells) is tightly regulated; each pre-existing centriole nucleates only one centriole as the cell prepares for division. The structure of centrioles is barrel-shaped, with a nine-fold symmetry of microtubules. This organization of microtubules is essential for the ancestral function of centriole⁻cilium nucleation. In animal cells, centrioles have gained an additional role: recruiting pericentriolar material (PCM) to form a centrosome. Therefore, it is striking that in animal spermatozoa, the centrioles have a remarkable diversity of structures, where some are so anomalous that they are referred to as atypical centrioles and are barely recognizable. The atypical centriole maintains the ability to form a centrosome and nucleate a new centriole, and therefore reveals the most rudimentary structure that is needed for centriole function. However, the atypical centriole appears to be incapable of forming a cilium. Here, we propose that the diversity in sperm centriole structure is due to rapid evolution in the shape of the spermatozoa head and neck. The enhanced diversity may be driven by a combination of direct selection for novel centriole functions and pleiotropy, which eliminates centriole properties that are dispensable in the spermatozoa function.
Keywords: centriole; centriole remodeling; centrosome; centrosome reduction; cilium; evolution; sperm; spermatogenesis.
Conflict of interest statement
The Authors declare no Competing Financial or Non-Financial Interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

