Modeling polypharmacy side effects with graph convolutional networks
- PMID: 29949996
- PMCID: PMC6022705
- DOI: 10.1093/bioinformatics/bty294
Modeling polypharmacy side effects with graph convolutional networks
Abstract
Motivation: The use of drug combinations, termed polypharmacy, is common to treat patients with complex diseases or co-existing conditions. However, a major consequence of polypharmacy is a much higher risk of adverse side effects for the patient. Polypharmacy side effects emerge because of drug-drug interactions, in which activity of one drug may change, favorably or unfavorably, if taken with another drug. The knowledge of drug interactions is often limited because these complex relationships are rare, and are usually not observed in relatively small clinical testing. Discovering polypharmacy side effects thus remains an important challenge with significant implications for patient mortality and morbidity.
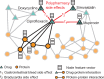
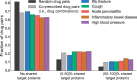
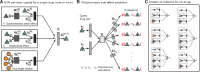
Results: Here, we present Decagon, an approach for modeling polypharmacy side effects. The approach constructs a multimodal graph of protein-protein interactions, drug-protein target interactions and the polypharmacy side effects, which are represented as drug-drug interactions, where each side effect is an edge of a different type. Decagon is developed specifically to handle such multimodal graphs with a large number of edge types. Our approach develops a new graph convolutional neural network for multirelational link prediction in multimodal networks. Unlike approaches limited to predicting simple drug-drug interaction values, Decagon can predict the exact side effect, if any, through which a given drug combination manifests clinically. Decagon accurately predicts polypharmacy side effects, outperforming baselines by up to 69%. We find that it automatically learns representations of side effects indicative of co-occurrence of polypharmacy in patients. Furthermore, Decagon models particularly well polypharmacy side effects that have a strong molecular basis, while on predominantly non-molecular side effects, it achieves good performance because of effective sharing of model parameters across edge types. Decagon opens up opportunities to use large pharmacogenomic and patient population data to flag and prioritize polypharmacy side effects for follow-up analysis via formal pharmacological studies.
Availability and implementation: Source code and preprocessed datasets are at: http://snap.stanford.edu/decagon.
Figures

References
-
- Bicker J. et al. (2017) Elucidation of the impact of p-glycoprotein and breast cancer resistance protein on the brain distribution of catechol-o-methyltransferase inhibitors. Drug Metab. Dispos., 45, 1282–1291. - PubMed
-
- Campillos M. et al. (2008) Drug target identification using side-effect similarity. Science, 321, 263–266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

