Gallid Herpesvirus 1 Initiates Apoptosis in Uninfected Cells through Paracrine Repression of p53
- PMID: 29950417
- PMCID: PMC6146683
- DOI: 10.1128/JVI.00529-18
Gallid Herpesvirus 1 Initiates Apoptosis in Uninfected Cells through Paracrine Repression of p53
Abstract
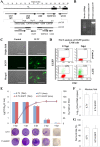
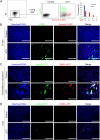
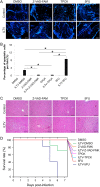
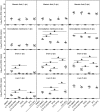
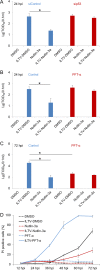
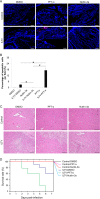
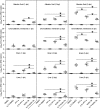
Apoptosis is a common innate defense mechanism of host cells against viral infection and is therefore suppressed by many viruses, including herpes simplex virus (HSV), via various strategies. A recent in vivo study reported the apoptosis of remote uninfected cells during Gallid herpesvirus 1 (GaHV-1) infection, yet little is known about this previously unknown aspect of herpesvirus-host interactions. The aim of the present study was to investigate the apoptosis of uninfected host cells during GaHV-1 infection. The present study used in vitro and in ovo models, which avoided potential interference by host antiviral immunity, and demonstrated that this GaHV-1-host interaction is independent of host immune responses and important for both the pathological effect of viral infection and early viral dissemination from the primary infection site to distant tissues. Further, we revealed that GaHV-1 infection triggers this process in a paracrine-regulated manner. Using genome-wide transcriptome analyses in combination with a set of functional studies, we found that this paracrine-regulated effect requires the repression of p53 activity in uninfected cells. In contrast, the activation of p53 not only prevented the apoptosis of remote uninfected cells and subsequent pathological damage induced by GaHV-1 infection but also delayed viral dissemination significantly. Moreover, p53 activation repressed viral replication both in vitro and in ovo, suggesting that dual cell-intrinsic mechanisms underlie the suppression of GaHV-1 infection by p53 activation. This study uncovers the mechanism underlying the herpesvirus-triggered apoptosis of remote host cells and extends our understanding of both herpesvirus-host interactions and the roles of p53 in viral infection.IMPORTANCE It is well accepted that herpesviruses suppress the apoptosis of host cells via various strategies to ensure sustained viral replication during infection. However, a recent in vivo study reported the apoptosis of remote uninfected cells during GaHV-1 infection. The mechanism and the biological meaning of this unexpected herpesvirus-host interaction are unclear. This study uncovers the mechanisms of herpesvirus-triggered apoptosis in uninfected cells and may also contribute to a mechanistic illustration of paracrine-regulated apoptosis induced by other viruses in uninfected host cells.
Keywords: alphaherpesviruses; p53; paracrine apoptosis; virus-host interactions.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Fos Facilitates Gallid Alpha-Herpesvirus 1 Infection by Transcriptional Control of Host Metabolic Genes and Viral Immediate Early Gene.Viruses. 2021 Jun 9;13(6):1110. doi: 10.3390/v13061110. Viruses. 2021. PMID: 34207926 Free PMC article.
-
Genome-Wide Gene Expression Analysis Identifies the Proto-oncogene Tyrosine-Protein Kinase Src as a Crucial Virulence Determinant of Infectious Laryngotracheitis Virus in Chicken Cells.J Virol. 2015 Oct 7;90(1):9-21. doi: 10.1128/JVI.01817-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26446601 Free PMC article.
-
Environmental pollutants modulate RNA and DNA virus-activated miRNA-155 expression and innate immune system responses: Insights into new immunomodulative mechanisms.J Immunotoxicol. 2020 Dec;17(1):86-93. doi: 10.1080/1547691X.2020.1740838. J Immunotoxicol. 2020. PMID: 32233818
-
Marek's disease: Genetic regulation of gallid herpesvirus 2 infection and latency.Vet J. 2015 Sep;205(3):339-48. doi: 10.1016/j.tvjl.2015.04.038. Epub 2015 May 7. Vet J. 2015. PMID: 26067852 Review.
-
The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine.Avian Pathol. 2017 Dec;46(6):585-593. doi: 10.1080/03079457.2017.1331029. Epub 2017 Jun 19. Avian Pathol. 2017. PMID: 28532159 Review.
Cited by
-
P53 maintains gallid alpha herpesvirus 1 replication by direct regulation of nucleotide metabolism and ATP synthesis through its target genes.Front Microbiol. 2022 Nov 24;13:1044141. doi: 10.3389/fmicb.2022.1044141. eCollection 2022. Front Microbiol. 2022. PMID: 36504811 Free PMC article.
-
Avian Flavivirus Infection of Monocytes/Macrophages by Extensive Subversion of Host Antiviral Innate Immune Responses.J Virol. 2019 Oct 29;93(22):e00978-19. doi: 10.1128/JVI.00978-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462573 Free PMC article.
-
Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition.J Virol. 2020 May 4;94(10):e02049-19. doi: 10.1128/JVI.02049-19. Print 2020 May 4. J Virol. 2020. PMID: 32161176 Free PMC article.
-
Fos Facilitates Gallid Alpha-Herpesvirus 1 Infection by Transcriptional Control of Host Metabolic Genes and Viral Immediate Early Gene.Viruses. 2021 Jun 9;13(6):1110. doi: 10.3390/v13061110. Viruses. 2021. PMID: 34207926 Free PMC article.
-
Immune Response to Herpes Simplex Virus Infection and Vaccine Development.Vaccines (Basel). 2020 Jun 12;8(2):302. doi: 10.3390/vaccines8020302. Vaccines (Basel). 2020. PMID: 32545507 Free PMC article. Review.
References
-
- Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. 2014. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A 111:15438–15443. doi:10.1073/pnas.1412767111. - DOI - PMC - PubMed
-
- Sabri F, Granath F, Hjalmarsson A, Aurelius E, Sköldenberg B. 2006. Modulation of sFas indicates apoptosis in human herpes simplex encephalitis. J Neuroimmunol 171:171–176. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

