Rubella Viruses Shift Cellular Bioenergetics to a More Oxidative and Glycolytic Phenotype with a Strain-Specific Requirement for Glutamine
- PMID: 29950419
- PMCID: PMC6096829
- DOI: 10.1128/JVI.00934-18
Rubella Viruses Shift Cellular Bioenergetics to a More Oxidative and Glycolytic Phenotype with a Strain-Specific Requirement for Glutamine
Abstract
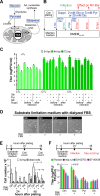
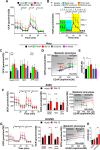
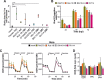
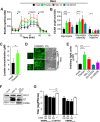
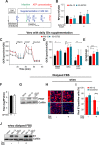
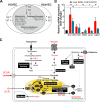
The flexible regulation of cellular metabolic pathways enables cellular adaptation to changes in energy demand under conditions of stress such as posed by a virus infection. To analyze such an impact on cellular metabolism, rubella virus (RV) was used in this study. RV replication under selected substrate supplementation with glucose, pyruvate, and glutamine as essential nutrients for mammalian cells revealed its requirement for glutamine. The assessment of the mitochondrial respiratory (based on the oxygen consumption rate) and glycolytic (based on the extracellular acidification rate) rate and capacity by respective stress tests through Seahorse technology enabled determination of the bioenergetic phenotype of RV-infected cells. Irrespective of the cellular metabolic background, RV infection induced a shift of the bioenergetic state of epithelial cells (Vero and A549) and human umbilical vein endothelial cells to a higher oxidative and glycolytic level. Interestingly there was a RV strain-specific, but genotype-independent demand for glutamine to induce a significant increase in metabolic activity. While glutaminolysis appeared to be rather negligible for RV replication, glutamine could serve as donor of its amide nitrogen in biosynthesis pathways for important metabolites. This study suggests that the capacity of RVs to induce metabolic alterations could evolve differently during natural infection. Thus, changes in cellular bioenergetics represent an important component of virus-host interactions and could complement our understanding of the viral preference for a distinct host cell population.IMPORTANCE RV pathologies, especially during embryonal development, could be connected with its impact on mitochondrial metabolism. With bioenergetic phenotyping we pursued a rather novel approach in virology. For the first time it was shown that a virus infection could shift the bioenergetics of its infected host cell to a higher energetic state. Notably, the capacity to induce such alterations varied among different RV isolates. Thus, our data add viral adaptation of cellular metabolic activity to its specific needs as a novel aspect to virus-host evolution. In addition, this study emphasizes the implementation of different viral strains in the study of virus-host interactions and the use of bioenergetic phenotyping of infected cells as a biomarker for virus-induced pathological alterations.
Keywords: 2-deoxyglucose; ECAR; OCR; extracellular acidification rate; extracellular flux analysis; glucose uptake; glutaminolysis; glycolysis; kynurenine pathway; metabolic phenotype; mitochondrial respiration; nucleotide biosynthesis; oxygen consumption rate; rubella virus.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling.Biochim Biophys Acta. 2014 Jan;1837(1):51-62. doi: 10.1016/j.bbabio.2013.07.008. Epub 2013 Jul 23. Biochim Biophys Acta. 2014. PMID: 23891695
-
A Novel Methodology for Bioenergetic Analysis of Plasmodium falciparum Reveals a Glucose-Regulated Metabolic Shift and Enables Mode of Action Analyses of Mitochondrial Inhibitors.ACS Infect Dis. 2016 Dec 9;2(12):903-916. doi: 10.1021/acsinfecdis.6b00101. Epub 2016 Oct 25. ACS Infect Dis. 2016. PMID: 27718558 Free PMC article.
-
Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements.J Biol Chem. 2017 Apr 28;292(17):7189-7207. doi: 10.1074/jbc.M116.774471. Epub 2017 Mar 7. J Biol Chem. 2017. PMID: 28270511 Free PMC article.
-
Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells.Int J Biochem Cell Biol. 2011 Jul;43(7):950-68. doi: 10.1016/j.biocel.2010.05.003. Epub 2010 May 10. Int J Biochem Cell Biol. 2011. PMID: 20460169 Review.
-
Viral activation of cellular metabolism.Virology. 2015 May;479-480:609-18. doi: 10.1016/j.virol.2015.02.038. Epub 2015 Mar 23. Virology. 2015. PMID: 25812764 Free PMC article. Review.
Cited by
-
Glycolysis Is an Intrinsic Factor for Optimal Replication of a Norovirus.mBio. 2019 Mar 12;10(2):e02175-18. doi: 10.1128/mBio.02175-18. mBio. 2019. PMID: 30862747 Free PMC article.
-
Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases.Microorganisms. 2020 Mar 11;8(3):388. doi: 10.3390/microorganisms8030388. Microorganisms. 2020. PMID: 32168836 Free PMC article. Review.
-
Impaired mitochondria of Tregs decreases OXPHOS-derived ATP in primary immune thrombocytopenia with positive plasma pathogens detected by metagenomic sequencing.Exp Hematol Oncol. 2022 Sep 1;11(1):48. doi: 10.1186/s40164-022-00304-y. Exp Hematol Oncol. 2022. PMID: 36050760 Free PMC article.
-
The Contribution of Phospholipase A2 and Metalloproteinases to the Synergistic Action of Viper Venom on the Bioenergetic Profile of Vero Cells.Toxins (Basel). 2022 Oct 23;14(11):724. doi: 10.3390/toxins14110724. Toxins (Basel). 2022. PMID: 36355974 Free PMC article. Review.
-
The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response.Biochimie. 2021 Jan;180:169-177. doi: 10.1016/j.biochi.2020.11.010. Epub 2020 Nov 12. Biochimie. 2021. PMID: 33189832 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

