A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV
- PMID: 29950421
- PMCID: PMC6146697
- DOI: 10.1128/JVI.00837-18
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV
Abstract
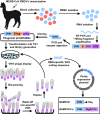
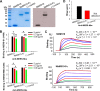
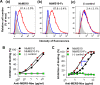
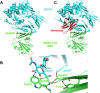
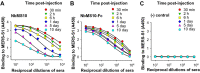
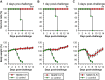
The newly emerged Middle East respiratory syndrome coronavirus (MERS-CoV) continues to infect humans and camels, calling for efficient, cost-effective, and broad-spectrum strategies to control its spread. Nanobodies (Nbs) are single-domain antibodies derived from camelids and sharks and are potentially cost-effective antivirals with small size and great expression yield. In this study, we developed a novel neutralizing Nb (NbMS10) and its human-Fc-fused version (NbMS10-Fc), both of which target the MERS-CoV spike protein receptor-binding domain (RBD). We further tested their receptor-binding affinity, recognizing epitopes, cross-neutralizing activity, half-life, and efficacy against MERS-CoV infection. Both Nbs can be expressed in yeasts with high yield, bind to MERS-CoV RBD with high affinity, and block the binding of MERS-CoV RBD to the MERS-CoV receptor. The binding site of the Nbs on the RBD was mapped to be around residue Asp539, which is part of a conserved conformational epitope at the receptor-binding interface. NbMS10 and NbMS10-Fc maintained strong cross-neutralizing activity against divergent MERS-CoV strains isolated from humans and camels. Particularly, NbMS10-Fc had significantly extended half-life in vivo; a single-dose treatment of NbMS10-Fc exhibited high prophylactic and therapeutic efficacy by completely protecting humanized mice from lethal MERS-CoV challenge. Overall, this study proves the feasibility of producing cost-effective, potent, and broad-spectrum Nbs against MERS-CoV and has produced Nbs with great potentials as anti-MERS-CoV therapeutics.IMPORTANCE Therapeutic development is critical for preventing and treating continual MERS-CoV infections in humans and camels. Because of their small size, nanobodies (Nbs) have advantages as antiviral therapeutics (e.g., high expression yield and robustness for storage and transportation) and also potential limitations (e.g., low antigen-binding affinity and fast renal clearance). Here, we have developed novel Nbs that specifically target the receptor-binding domain (RBD) of MERS-CoV spike protein. They bind to a conserved site on MERS-CoV RBD with high affinity, blocking RBD's binding to MERS-CoV receptor. Through engineering a C-terminal human Fc tag, the in vivo half-life of the Nbs is significantly extended. Moreover, the Nbs can potently cross-neutralize the infections of diverse MERS-CoV strains isolated from humans and camels. The Fc-tagged Nb also completely protects humanized mice from lethal MERS-CoV challenge. Taken together, our study has discovered novel Nbs that hold promise as potent, cost-effective, and broad-spectrum anti-MERS-CoV therapeutic agents.
Keywords: MERS-CoV; cross-neutralization; nanobody; protective efficacy; receptor-binding domain; spike protein.
Copyright © 2018 Zhao et al.
Figures


Similar articles
-
Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article.
-
Enhanced Ability of Oligomeric Nanobodies Targeting MERS Coronavirus Receptor-Binding Domain.Viruses. 2019 Feb 19;11(2):166. doi: 10.3390/v11020166. Viruses. 2019. PMID: 30791410 Free PMC article.
-
A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein.Cell Res. 2015 Nov;25(11):1237-49. doi: 10.1038/cr.2015.113. Epub 2015 Sep 22. Cell Res. 2015. PMID: 26391698 Free PMC article.
-
Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain.Viruses. 2019 Jan 14;11(1):60. doi: 10.3390/v11010060. Viruses. 2019. PMID: 30646569 Free PMC article. Review.
-
Neutralizing Monoclonal Antibodies as Promising Therapeutics against Middle East Respiratory Syndrome Coronavirus Infection.Viruses. 2018 Nov 30;10(12):680. doi: 10.3390/v10120680. Viruses. 2018. PMID: 30513619 Free PMC article. Review.
Cited by
-
SARS-CoV-2 neutralizing antibody development strategies.Turk J Biol. 2020 Jun 21;44(3):203-214. doi: 10.3906/biy-2005-91. eCollection 2020. Turk J Biol. 2020. PMID: 32595357 Free PMC article.
-
Combining a Fusion Inhibitory Peptide Targeting the MERS-CoV S2 Protein HR1 Domain and a Neutralizing Antibody Specific for the S1 Protein Receptor-Binding Domain (RBD) Showed Potent Synergism against Pseudotyped MERS-CoV with or without Mutations in RBD.Viruses. 2019 Jan 6;11(1):31. doi: 10.3390/v11010031. Viruses. 2019. PMID: 30621343 Free PMC article.
-
HRP-conjugated-nanobody-based cELISA for rapid and sensitive clinical detection of ASFV antibodies.Appl Microbiol Biotechnol. 2022 Jun;106(11):4269-4285. doi: 10.1007/s00253-022-11981-4. Epub 2022 May 25. Appl Microbiol Biotechnol. 2022. PMID: 35612629 Free PMC article.
-
Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection.Emerg Microbes Infect. 2019;8(1):760-772. doi: 10.1080/22221751.2019.1620083. Emerg Microbes Infect. 2019. PMID: 31130102 Free PMC article.
-
Nanobodies as powerful pulmonary targeted biotherapeutics against SARS-CoV-2, pharmaceutical point of view.Biochim Biophys Acta Gen Subj. 2021 Nov;1865(11):129974. doi: 10.1016/j.bbagen.2021.129974. Epub 2021 Jul 31. Biochim Biophys Acta Gen Subj. 2021. PMID: 34343644 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

