A Roma founder BIN1 mutation causes a novel phenotype of centronuclear myopathy with rigid spine
- PMID: 29950440
- PMCID: PMC6070382
- DOI: 10.1212/WNL.0000000000005862
A Roma founder BIN1 mutation causes a novel phenotype of centronuclear myopathy with rigid spine
Abstract
Objective: To describe a large series of BIN1 patients, in which a novel founder mutation in the Roma population of southern Spain has been identified.
Methods: Patients diagnosed with centronuclear myopathy (CNM) at 5 major reference centers for neuromuscular disease in Spain (n = 53) were screened for BIN1 mutations. Clinical, histologic, radiologic, and genetic features were analyzed.
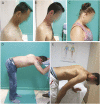
Results: Eighteen patients from 13 families carried the p.Arg234Cys variant; 16 of them were homozygous for it and 2 had compound heterozygous p.Arg234Cys/p.Arg145Cys mutations. Both BIN1 variants have only been identified in Roma, causing 100% of CNM in this ethnic group in our cohort. The haplotype analysis confirmed all families are related. In addition to clinical features typical of CNM, such as proximal limb weakness and ophthalmoplegia, most patients in our cohort presented with prominent axial weakness, often associated with rigid spine. Severe fat replacement of paravertebral muscles was demonstrated by muscle imaging. This phenotype seems to be specific to the p.Arg234Cys mutation, not reported in other BIN1 mutations. Extreme clinical variability was observed in the 2 compound heterozygous patients for the p.Arg234Cys/p.Arg145Cys mutations, from a congenital onset with catastrophic outcome to a late-onset disease. Screening of European Roma controls (n = 758) for the p.Arg234Cys variant identified a carrier frequency of 3.5% among the Spanish Roma.
Conclusion: We have identified a BIN1 founder Roma mutation associated with a highly specific phenotype, which is, from the present cohort, the main cause of CNM in Spain.
© 2018 American Academy of Neurology.
Figures



Similar articles
-
Dominant Centronuclear Myopathy with Early Childhood Onset due to a Novel Mutation in BIN1.J Neuromuscul Dis. 2017;4(4):349-355. doi: 10.3233/JND-170238. J Neuromuscul Dis. 2017. PMID: 29103045
-
Adult-onset autosomal dominant centronuclear myopathy due to BIN1 mutations.Brain. 2014 Dec;137(Pt 12):3160-70. doi: 10.1093/brain/awu272. Epub 2014 Sep 25. Brain. 2014. PMID: 25260562
-
Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies.Acta Neuropathol. 2011 Feb;121(2):253-66. doi: 10.1007/s00401-010-0754-2. Epub 2010 Oct 7. Acta Neuropathol. 2011. PMID: 20927630
-
Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation.Orphanet J Rare Dis. 2010 Dec 3;5:35. doi: 10.1186/1750-1172-5-35. Orphanet J Rare Dis. 2010. PMID: 21129173 Free PMC article. Review.
-
Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2.J Struct Biol. 2016 Oct;196(1):37-47. doi: 10.1016/j.jsb.2016.06.015. Epub 2016 Jun 23. J Struct Biol. 2016. PMID: 27343996 Free PMC article. Review.
Cited by
-
Update on Congenital Myopathies in Adulthood.Int J Mol Sci. 2020 May 24;21(10):3694. doi: 10.3390/ijms21103694. Int J Mol Sci. 2020. PMID: 32456280 Free PMC article. Review.
-
Mice with muscle-specific deletion of Bin1 recapitulate centronuclear myopathy and acute downregulation of dynamin 2 improves their phenotypes.Mol Ther. 2022 Feb 2;30(2):868-880. doi: 10.1016/j.ymthe.2021.08.006. Epub 2021 Aug 8. Mol Ther. 2022. PMID: 34371181 Free PMC article.
-
NOVEL intronic CAPN3 Roma mutation alters splicing causing RNA mediated decay.Ann Clin Transl Neurol. 2019 Nov;6(11):2328-2333. doi: 10.1002/acn3.50910. Epub 2019 Oct 14. Ann Clin Transl Neurol. 2019. PMID: 31612648 Free PMC article.
-
Dynamics of membrane tubulation coupled with fission by a two-component module.Proc Natl Acad Sci U S A. 2024 May 14;121(20):e2402180121. doi: 10.1073/pnas.2402180121. Epub 2024 May 8. Proc Natl Acad Sci U S A. 2024. PMID: 38717859 Free PMC article.
-
Common Pathogenic Mechanisms in Centronuclear and Myotubular Myopathies and Latest Treatment Advances.Int J Mol Sci. 2021 Oct 21;22(21):11377. doi: 10.3390/ijms222111377. Int J Mol Sci. 2021. PMID: 34768808 Free PMC article. Review.
References
-
- Claeys KG, Maisonobe T, Bohm J, et al. . Phenotype of a patient with recessive centronuclear myopathy and a novel BIN1 mutation. Neurology 2010;74:519–521. - PubMed
-
- Nicot AS, Toussaint A, Tosch V, et al. . Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 2007;39:1134–1139. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
