Study protocol for a multicentre, cluster randomised, superiority trial evaluating the impact of computerised decision support, audit and feedback on antibiotic use: the COMPuterized Antibiotic Stewardship Study (COMPASS)
- PMID: 29950480
- PMCID: PMC6042555
- DOI: 10.1136/bmjopen-2018-022666
Study protocol for a multicentre, cluster randomised, superiority trial evaluating the impact of computerised decision support, audit and feedback on antibiotic use: the COMPuterized Antibiotic Stewardship Study (COMPASS)
Abstract
Introduction: Inappropriate use of antimicrobials in hospitals contributes to antimicrobial resistance. Antimicrobial stewardship (AMS) interventions aim to improve antimicrobial prescribing, but they are often resource and personnel intensive. Computerised decision supportsystems (CDSSs) seem a promising tool to improve antimicrobial prescribing but have been insufficiently studied in clinical trials.
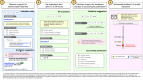
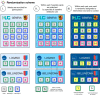
Methods and analysis: The COMPuterized Antibiotic Stewardship Study trial, is a publicly funded, open-label, cluster randomised, controlled superiority trial which aims to determine whether a multimodal CDSS intervention integrated in the electronic health record (EHR) reduces overall antibiotic exposure in adult patients hospitalised in wards of two secondary and one tertiary care centre in Switzerland compared with 'standard-of-care' AMS. Twenty-four hospital wards will be randomised 1:1 to either intervention or control, using a 'pair-matching' approach based on baseline antibiotic use, specialty and centre. The intervention will consist of (1) decision support for the choice of antimicrobial treatment and duration of treatment for selected indications (based on indication entry), (2) accountable justification for deviation from the local guidelines (with regard to the choice of molecules and duration), (3) alerts for self-guided re-evaluation of treatment on calendar day 4 of antimicrobial therapy and (4) monthly ward-level feedback of antimicrobial prescribing indicators. The primary outcome will be the difference in overall systemic antibiotic use measured in days of therapy per admission based on administration data recorded in the EHR over the whole intervention period (12 months), taking into account clustering. Secondary outcomes include qualitative and quantitative antimicrobial use indicators, economic outcomes and clinical, microbiological and patient safety indicators.
Ethics and dissemination: Ethics approval was obtained for all participating sites (Comission Cantonale d'Éthique de la Recherche (CCER)2017-00454). The results of the trial will be submitted for publication in a peer-reviewed journal. Further dissemination activities will be presentations/posters at national and international conferences.
Trial registration number: NCT03120975; Pre-results.
Keywords: antimicrobial stewardship; cluster randomised trial; computerised decision support system.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Impact of interactive computerised decision support for hospital antibiotic use (COMPASS): an open-label, cluster-randomised trial in three Swiss hospitals.Lancet Infect Dis. 2022 Oct;22(10):1493-1502. doi: 10.1016/S1473-3099(22)00308-5. Epub 2022 Jul 20. Lancet Infect Dis. 2022. PMID: 35870478 Free PMC article. Clinical Trial.
-
Open-label, single-centre, cluster-randomised controlled trial to Evaluate the Potential Impact of Computerisedantimicrobial stewardship (EPIC) on the antimicrobial use after cardiovascular surgeries: EPIC trial study original protocol.BMJ Open. 2020 Nov 26;10(11):e039717. doi: 10.1136/bmjopen-2020-039717. BMJ Open. 2020. PMID: 33243799 Free PMC article.
-
Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial.BMJ. 2019 Feb 12;364:l236. doi: 10.1136/bmj.l236. BMJ. 2019. PMID: 30755451 Free PMC article. Clinical Trial.
-
The impact of antimicrobial stewardship strategies on antibiotic appropriateness and prescribing behaviours in selected countries in the Middle East: a systematic review.East Mediterr Health J. 2017 Aug 20;23(6):430-440. doi: 10.26719/2017.23.6.430. East Mediterr Health J. 2017. PMID: 28836656
-
Audit and Feedback Interventions for Antibiotic Prescribing in Primary Care: A Systematic Review and Meta-analysis.Clin Infect Dis. 2025 Feb 24;80(2):253-262. doi: 10.1093/cid/ciae604. Clin Infect Dis. 2025. PMID: 39657007 Free PMC article.
Cited by
-
Impact of interactive computerised decision support for hospital antibiotic use (COMPASS): an open-label, cluster-randomised trial in three Swiss hospitals.Lancet Infect Dis. 2022 Oct;22(10):1493-1502. doi: 10.1016/S1473-3099(22)00308-5. Epub 2022 Jul 20. Lancet Infect Dis. 2022. PMID: 35870478 Free PMC article. Clinical Trial.
-
Effective Antimicrobial StewaRdship StrategIES (ARIES): Cluster Randomized Trial of Computerized Decision Support System and Prospective Review and Feedback.Open Forum Infect Dis. 2020 Jun 29;7(7):ofaa254. doi: 10.1093/ofid/ofaa254. eCollection 2020 Jul. Open Forum Infect Dis. 2020. PMID: 32704514 Free PMC article.
-
How to Develop and Implement a Computerized Decision Support System Integrated for Antimicrobial Stewardship? Experiences From Two Swiss Hospital Systems.Front Digit Health. 2021 Feb 16;2:583390. doi: 10.3389/fdgth.2020.583390. eCollection 2020. Front Digit Health. 2021. PMID: 34713055 Free PMC article.
-
Machine Learning and Antibiotic Management.Antibiotics (Basel). 2022 Feb 24;11(3):304. doi: 10.3390/antibiotics11030304. Antibiotics (Basel). 2022. PMID: 35326768 Free PMC article.
References
-
- Baur D, Gladstone BP, Burkert F, et al. . Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017;17:990–1001. 10.1016/S1473-3099(17)30325-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
