Proton irradiation orchestrates macrophage reprogramming through NFκB signaling
- PMID: 29950610
- PMCID: PMC6021396
- DOI: 10.1038/s41419-018-0757-9
Proton irradiation orchestrates macrophage reprogramming through NFκB signaling
Abstract
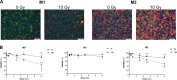
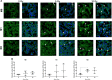
Tumor-associated macrophages (TAMs) represent potential targets for anticancer treatments as these cells play critical roles in tumor progression and frequently antagonize the response to treatments. TAMs are usually associated to an M2-like phenotype, characterized by anti-inflammatory and protumoral properties. This phenotype contrasts with the M1-like macrophages, which exhibits proinflammatory, phagocytic, and antitumoral functions. As macrophages hold a high plasticity, strategies to orchestrate the reprogramming of M2-like TAMs towards a M1 antitumor phenotype offer potential therapeutic benefits. One of the most used anticancer treatments is the conventional X-ray radiotherapy (RT), but this therapy failed to reprogram TAMs towards an M1 phenotype. While protontherapy is more and more used in clinic to circumvent the side effects of conventional RT, the effects of proton irradiation on macrophages have not been investigated yet. Here we showed that M1 macrophages (THP-1 cell line) were more resistant to proton irradiation than unpolarized (M0) and M2 macrophages, which correlated with differential DNA damage detection. Moreover, proton irradiation-induced macrophage reprogramming from M2 to a mixed M1/M2 phenotype. This reprogramming required the nuclear translocation of NFκB p65 subunit as the inhibition of IκBα phosphorylation completely reverted the macrophage re-education. Altogether, the results suggest that proton irradiation promotes NFκB-mediated macrophage polarization towards M1 and opens new perspectives for macrophage targeting with charged particle therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
M1 Macrophages but Not M2 Macrophages Are Characterized by Upregulation of CRP Expression via Activation of NFκB: a Possible Role for Ox-LDL in Macrophage Polarization.Inflammation. 2018 Aug;41(4):1477-1487. doi: 10.1007/s10753-018-0793-8. Inflammation. 2018. PMID: 29687414
-
Expression of chemokines in macrophage polarization and downregulation of NFκB in aorta allow macrophage polarization by diosgenin in atherosclerosis.J Biochem Mol Toxicol. 2020 Feb;34(2):e22422. doi: 10.1002/jbt.22422. Epub 2019 Nov 15. J Biochem Mol Toxicol. 2020. PMID: 31729780
-
Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies.Front Immunol. 2017 Jul 14;8:828. doi: 10.3389/fimmu.2017.00828. eCollection 2017. Front Immunol. 2017. PMID: 28769933 Free PMC article. Review.
-
Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer.Int J Radiat Oncol Biol Phys. 2018 Mar 15;100(4):1034-1043. doi: 10.1016/j.ijrobp.2017.11.043. Epub 2017 Dec 7. Int J Radiat Oncol Biol Phys. 2018. PMID: 29485045
-
Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage "Switch" Phenotype.Biomed Res Int. 2015;2015:341308. doi: 10.1155/2015/341308. Epub 2015 Aug 23. Biomed Res Int. 2015. PMID: 26366410 Free PMC article. Review.
Cited by
-
NF-κB/TWIST1 Mediates Migration and Phagocytosis of Macrophages in the Mice Model of Implant-Associated Staphylococcus aureus Osteomyelitis.Front Microbiol. 2020 Jun 12;11:1301. doi: 10.3389/fmicb.2020.01301. eCollection 2020. Front Microbiol. 2020. PMID: 32595631 Free PMC article.
-
Thioredoxin Reductase Activity Predicts Gold Nanoparticle Radiosensitization Effect.Nanomaterials (Basel). 2019 Feb 19;9(2):295. doi: 10.3390/nano9020295. Nanomaterials (Basel). 2019. PMID: 30791480 Free PMC article.
-
Could Protons and Carbon Ions Be the Silver Bullets Against Pancreatic Cancer?Int J Mol Sci. 2020 Jul 4;21(13):4767. doi: 10.3390/ijms21134767. Int J Mol Sci. 2020. PMID: 32635552 Free PMC article. Review.
-
Niosomes as Biocompatible Scaffolds for the Multivalent Presentation of Tumor-Associated Antigens (TACAs) to the Immune System.Bioconjug Chem. 2023 Jan 18;34(1):181-192. doi: 10.1021/acs.bioconjchem.2c00383. Epub 2022 Dec 15. Bioconjug Chem. 2023. PMID: 36519843 Free PMC article.
-
Neutrophil Metabolic Shift during their Lifecycle: Impact on their Survival and Activation.Int J Mol Sci. 2019 Dec 31;21(1):287. doi: 10.3390/ijms21010287. Int J Mol Sci. 2019. PMID: 31906243 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

