Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche
- PMID: 29950724
- PMCID: PMC6042247
- DOI: 10.1038/s41586-018-0257-1
Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche
Erratum in
-
Publisher Correction: Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche.Nature. 2018 Oct;562(7727):E22. doi: 10.1038/s41586-018-0370-1. Nature. 2018. PMID: 30013120
Abstract
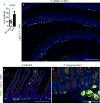
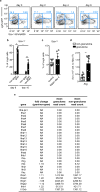
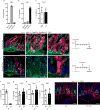
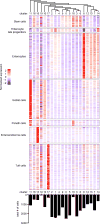
Epithelial surfaces form critical barriers to the outside world and are continuously renewed by adult stem cells1. Whereas dynamics of epithelial stem cells during homeostasis are increasingly well understood, how stem cells are redirected from a tissue-maintenance program to initiate repair after injury remains unclear. Here we examined infection by Heligmosomoides polygyrus, a co-evolved pathosymbiont of mice, to assess the epithelial response to disruption of the mucosal barrier. H. polygyrus disrupts tissue integrity by penetrating the duodenal mucosa, where it develops while surrounded by a multicellular granulomatous infiltrate2. Crypts overlying larvae-associated granulomas did not express intestinal stem cell markers, including Lgr53, in spite of continued epithelial proliferation. Granuloma-associated Lgr5- crypt epithelium activated an interferon-gamma (IFN-γ)-dependent transcriptional program, highlighted by Sca-1 expression, and IFN-γ-producing immune cells were found in granulomas. A similar epithelial response accompanied systemic activation of immune cells, intestinal irradiation, or ablation of Lgr5+ intestinal stem cells. When cultured in vitro, granuloma-associated crypt cells formed spheroids similar to those formed by fetal epithelium, and a sub-population of H. polygyrus-induced cells activated a fetal-like transcriptional program, demonstrating that adult intestinal tissues can repurpose aspects of fetal development. Therefore, re-initiation of the developmental program represents a fundamental mechanism by which the intestinal crypt can remodel itself to sustain function after injury.
Figures








Comment in
-
Intestinal Crypts Assume the Fetal Position in Response to Injury.Cell Stem Cell. 2018 Aug 2;23(2):158-159. doi: 10.1016/j.stem.2018.07.013. Cell Stem Cell. 2018. PMID: 30075126
Similar articles
-
Acetate, a metabolic product of Heligmosomoides polygyrus, facilitates intestinal epithelial barrier breakdown in a FFAR2-dependent manner.Int J Parasitol. 2022 Aug;52(9):591-601. doi: 10.1016/j.ijpara.2022.04.004. Epub 2022 Jun 6. Int J Parasitol. 2022. PMID: 35671792
-
Tracing the origin of adult intestinal stem cells.Nature. 2019 Jun;570(7759):107-111. doi: 10.1038/s41586-019-1212-5. Epub 2019 May 15. Nature. 2019. PMID: 31092921 Free PMC article.
-
Simultaneous real-time analysis of Paneth cell and intestinal stem cell response to interferon-γ by a novel stem cell niche tracking method.Biochem Biophys Res Commun. 2021 Mar 19;545:14-19. doi: 10.1016/j.bbrc.2021.01.050. Epub 2021 Jan 30. Biochem Biophys Res Commun. 2021. PMID: 33529805
-
Regulation of intestinal stem cell fate specification.Sci China Life Sci. 2015 Jun;58(6):570-8. doi: 10.1007/s11427-015-4859-7. Epub 2015 May 8. Sci China Life Sci. 2015. PMID: 25951932 Review.
-
Helminths' therapeutic potential to treat intestinal barrier dysfunction.Allergy. 2023 Nov;78(11):2892-2905. doi: 10.1111/all.15812. Epub 2023 Jul 14. Allergy. 2023. PMID: 37449458 Review.
Cited by
-
TGFB1 Induces Fetal Reprogramming and Enhances Intestinal Regeneration.bioRxiv [Preprint]. 2023 Jan 13:2023.01.13.523825. doi: 10.1101/2023.01.13.523825. bioRxiv. 2023. Update in: Cell Stem Cell. 2023 Nov 2;30(11):1520-1537.e8. doi: 10.1016/j.stem.2023.09.015. PMID: 36711781 Free PMC article. Updated. Preprint.
-
Enterocyte-innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium.Mucosal Immunol. 2022 Feb;15(2):362-372. doi: 10.1038/s41385-021-00468-6. Epub 2021 Nov 8. Mucosal Immunol. 2022. PMID: 34750455 Free PMC article.
-
p53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis.Nat Commun. 2022 May 23;13(1):2866. doi: 10.1038/s41467-022-30382-9. Nat Commun. 2022. PMID: 35606354 Free PMC article.
-
Tissue Homeostasis and Non-Homeostasis: From Cell Life Cycles to Organ States.Annu Rev Cell Dev Biol. 2022 Oct 6;38:395-418. doi: 10.1146/annurev-cellbio-120420-114855. Epub 2022 Jul 18. Annu Rev Cell Dev Biol. 2022. PMID: 35850152 Free PMC article. Review.
-
Good Neighbors: The Niche that Fine Tunes Mammalian Intestinal Regeneration.Cold Spring Harb Perspect Biol. 2022 May 27;14(5):a040865. doi: 10.1101/cshperspect.a040865. Cold Spring Harb Perspect Biol. 2022. PMID: 34580119 Free PMC article. Review.
References
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

