Efficacy and safety of four doses of glycopyrrolate/formoterol fumarate delivered via a metered dose inhaler compared with the monocomponents in patients with moderate-to-severe COPD
- PMID: 29950826
- PMCID: PMC6016010
- DOI: 10.2147/COPD.S166455
Efficacy and safety of four doses of glycopyrrolate/formoterol fumarate delivered via a metered dose inhaler compared with the monocomponents in patients with moderate-to-severe COPD
Abstract
Purpose: To determine the efficacy and safety of glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI 36/9.6, 36/7.2, 18/9.6, 9/9.6 µg) using innovative co-suspension delivery technology, compared with glycopyrrolate (GP) MDI 36 µg and formoterol fumarate (FF) MDI 9.6 µg, in patients with moderate-to-severe COPD.
Methods: In this Phase IIb, randomized, double-blind, balanced incomplete-block, two-period, cross-over study (NCT01349816), patients received treatment twice-daily for 7 days. The primary efficacy endpoint was forced expiratory volume in 1 second (FEV1) area under the curve from 0 to 12 hours (AUC0-12) on Day 7. Secondary efficacy endpoints were peak change from baseline in FEV1 through 2 hours; time to onset of action (≥10% improvement in mean FEV1); proportion of patients achieving ≥12% improvement in FEV1 on Day 1; peak change from baseline in inspiratory capacity (IC) on Days 1 and 7; change from baseline in morning pre-dose FEV1; peak change from baseline in FEV1 through 6 hours; and change from baseline in mean evening 12-hour post-dose trough FEV1 on Day 7. Safety was assessed.
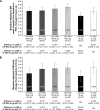
Results: All 185 randomized patients received treatment. All doses of GFF MDI significantly improved the primary endpoint compared with GP MDI 36 µg (all P≤0.0137). For peak change in FEV1 and IC and time to onset of action secondary endpoints, ≥2 doses of GFF MDI demonstrated superiority to GP MDI 36 µg. No significant differences were observed between GFF MDI and FF MDI 9.6 µg for primary and secondary endpoints. The incidence of adverse events was similar between treatments.
Conclusion: While all doses of GFF MDI were superior to GP MDI 36 µg for the primary end-point, in this study neither superiority of GFF MDI to FF MDI 9.6 µg nor a clear dose-response was observed. All treatments were well tolerated with no unexpected safety findings.
Keywords: LAMA/LABA; bronchodilator; chronic obstructive pulmonary disease; co-suspension delivery technology; fixed-dose combination; long-acting muscarinic antagonist; long-acting β2-agonist.
Conflict of interest statement
Disclosure CR is Chief Executive Officer of Pearl – a member of the AstraZeneca Group, and an employee of AstraZeneca. JP has received speaking fees from AstraZeneca, Genentech, Pfizer, and others, and has conducted trials on behalf of Boehringer Ingelheim, Forest, GlaxoSmithKline, Merck, Novartis, Sunovion, Teva, and others. EMK has served on advisory boards, speaker panels, or received travel reimbursement from Amphastar, AstraZeneca, Forest, Mylan, Novartis, Oriel, Pearl – a member of the AstraZeneca Group, Sunovion, Teva, and Theravance. He has conducted multicenter clinical trials for ~40 pharmaceutical companies. ESR and PD are employees of Pearl – a member of the AstraZeneca Group. The authors report no other conflicts of interest in this work.
Figures




References
-
- GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. - PMC - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018. [Accessed May 5, 2018]. Available from: http://www.goldcopd.org.
-
- Vehring R, Lechuga-Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28(42):15015–15023. - PubMed
-
- Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19(2):837–844. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

