The two faces of pannexins: new roles in inflammation and repair
- PMID: 29950881
- PMCID: PMC6016592
- DOI: 10.2147/JIR.S128401
The two faces of pannexins: new roles in inflammation and repair
Abstract
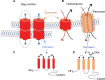
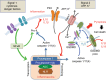
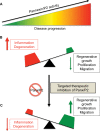
Pannexins belong to a family of ATP-release channels expressed in almost all cell types. An increasing body of literature on pannexins suggests that these channels play dual and sometimes contradictory roles, contributing to normal cell function, as well as to the pathological progression of disease. In this review, we summarize our understanding of pannexin "protective" and "harmful" functions in inflammation, regeneration and mechanical signaling. We also suggest a possible basis for pannexin's dual roles, related to extracellular ATP and K+ levels and the activation of various types of P2 receptors that are associated with pannexin. Finally, we speculate upon therapeutic strategies related to pannexin using eyes, lacrimal glands, and peripheral nerves as examples of interesting therapeutic targets.
Keywords: ATP; Panx1; inflammation; pannexin; purinergic signaling; regeneration.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

