Perioperative and Survival Outcomes Following Neoadjuvant FOLFIRINOX versus Gemcitabine Abraxane in Patients with Pancreatic Adenocarcinoma
- PMID: 29950957
- PMCID: PMC6018015
Perioperative and Survival Outcomes Following Neoadjuvant FOLFIRINOX versus Gemcitabine Abraxane in Patients with Pancreatic Adenocarcinoma
Abstract
Context: Neoadjuvant chemotherapy is increasingly used in borderline resectable and locally advanced pancreatic cancer to facilitate surgical resection.
Objective: To compare progression free survival and overall survival in patients receiving neoadjuvant FOLFIRINOX with those receiving gemcitabine/abraxane.
Design: Retrospective cohort study.
Setting: University of Colorado Hospital from 2012-2016.
Participants: Patients with pancreatic adenocarcinoma.
Interventions: Neoadjuvant FOLFIRINOX or gemcitabine/abraxane.
Outcome measures: Perioperative outcomes, progression free survival, and overall survival were compared between groups. A multivariate Cox proportional hazard model was applied to evaluate survival outcomes.
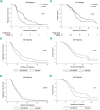
Results: We identified 120 patients: 83 (69.2%) FOLFIRINOX and 37 (30.8%) gemcitabine/abraxane. The FOLIFRINOX group was younger and had a lower ECOG performance status (p<0.05). Patients in the FOLFIRINOX group were more likely to undergo surgical resection compared to gemcitabine/abraxane (66.3% vs. 32.4%, p=0.002). Among all patients, median follow up was 16.9 months and FOLFIRINOX was associated with improved PFS (15.3 vs. 8.2 months, p=0.006), but not overall survival (23.5 vs. 18.7 months, p=0.228). In these patients, insulin-dependent diabetes was associated with a worse progression free survival and overall survival and surgical resection was protective. Among surgically resected patients, median follow up was 21.1 months and there was no difference in progression free survival (19.5 vs. 15.1 months) or overall survival (27.4 vs. 19.8 months) between the FOLFIRINOX and gemcitabine/abraxane groups, respectively (p>0.05). Insulin-dependent diabetes and a poor-to-moderate pathologic response was associated with worse progression free survival and overall survival.
Conclusion: Neoadjuvant FOLFIRINOX may improve progression free survival by increasing the proportion of patients undergoing surgical resection. Improved understanding of the role for selection bias and longer follow up are needed to better define the impact of neoadjuvant FOLFIRINOX on overall survival.
Keywords: Disease-Free Survival; Neoadjuvant Therapy; Pancreatic cancer; Survival; adult.
Conflict of interest statement
Conflict of Interest Authors are declared that there is no conflict of Interest.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Network NCC. NCCN guidelines version 1.2017. Pancreatic Adenocarcinoma. 2017
-
- Ryan DP, M H. In: Initial chemotherapy and radiation for nonmetastatic locally advanced unresectable and borderline resectable exocrine pancreatic cancer. Goldberg RMAS, Willett CG, Savarese DMF, editors. Up To Date.
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
