Alzheimer's Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits
- PMID: 29950970
- PMCID: PMC6008657
- DOI: 10.3389/fnins.2018.00383
Alzheimer's Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits
Abstract
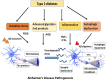
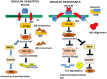
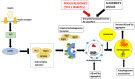
Alzheimer's disease (AD) and Type 2 Diabetes Mellitus (T2DM) are two of the most prevalent diseases in the elderly population worldwide. A growing body of epidemiological studies suggest that people with T2DM are at a higher risk of developing AD. Likewise, AD brains are less capable of glucose uptake from the surroundings resembling a condition of brain insulin resistance. Pathologically AD is characterized by extracellular plaques of Aβ and intracellular neurofibrillary tangles of hyperphosphorylated tau. T2DM, on the other hand is a metabolic disorder characterized by hyperglycemia and insulin resistance. In this review we have discussed how Insulin resistance in T2DM directly exacerbates Aβ and tau pathologies and elucidated the pathophysiological traits of synaptic dysfunction, inflammation, and autophagic impairments that are common to both diseases and indirectly impact Aβ and tau functions in the neurons. Elucidation of the underlying pathways that connect these two diseases will be immensely valuable for designing novel drug targets for Alzheimer's disease.
Keywords: abeta oligomers; autophagy; inflammation; insulin resistance; synaptic dysfunction; tau proteins.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

