Transplantation of Neural Precursor Cells Attenuates Chronic Immune Environment in Cervical Spinal Cord Injury
- PMID: 29951030
- PMCID: PMC6008566
- DOI: 10.3389/fneur.2018.00428
Transplantation of Neural Precursor Cells Attenuates Chronic Immune Environment in Cervical Spinal Cord Injury
Abstract
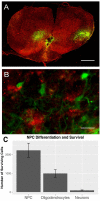
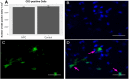
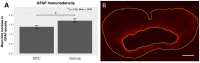
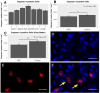
Inflammation after traumatic spinal cord injury (SCI) is non-resolving and thus still present in chronic injury stages. It plays a key role in the pathophysiology of SCI and has been associated with further neurodegeneration and development of neuropathic pain. Neural precursor cells (NPCs) have been shown to reduce the acute and sub-acute inflammatory response after SCI. In the present study, we examined effects of NPC transplantation on the immune environment in chronic stages of SCI. SCI was induced in rats by clip-compression of the cervical spinal cord at the level C6-C7. NPCs were transplanted 10 days post-injury. The functional outcome was assessed weekly for 8 weeks using the Basso, Beattie, and Bresnahan scale, the CatWalk system, and the grid walk test. Afterwards, the rats were sacrificed, and spinal cord sections were examined for M1/M2 macrophages, T lymphocytes, astrogliosis, and apoptosis using immunofluorescence staining. Rats treated with NPCs had compared to the control group significantly fewer pro-inflammatory M1 macrophages and reduced immunodensity for inducible nitric oxide synthase (iNOS), their marker enzyme. Anti-inflammatory M2 macrophages were rarely present 8 weeks after the SCI. In this model, the sub-acute transplantation of NPCs did not support survival and proliferation of M2 macrophages. Post-traumatic apoptosis, however, was significantly reduced in the NPC group, which might be explained by the altered microenvironment following NPC transplantation. Corresponding to these findings, reactive astrogliosis was significantly reduced in NPC-transplanted animals. Furthermore, we could observe a trend toward smaller cavity sizes and functional improvement following NPC transplantation. Our data suggest that transplantation of NPCs following SCI might attenuate inflammation even in chronic injury stages. This might prevent further neurodegeneration and could also set a stage for improved neuroregeneration after SCI.
Keywords: apoptosis; chronic inflammation; macrophages; neural precursor cells; neuroregeneration; spinal cord injury; stem cells.
Figures




References
-
- National Spinal Cord Injury Statistical Center Annual Statistical Report (2016).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

