Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide
- PMID: 29951043
- PMCID: PMC6008375
- DOI: 10.3389/fmicb.2018.01213
Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide
Abstract
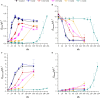
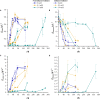
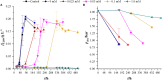
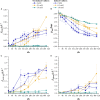
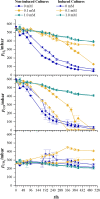
Cyanide is a minor constituent of crude syngas whose content depends on the feedstock and gasification procedure. It is a known poison to metal catalysts and inhibits iron-containing enzymes like carbon monoxide dehydrogenase of acetogenic organisms. Therefore, it is considered a component that has to be removed from the gas stream prior to use in chemical synthesis or syngas fermentation. We show that the growth rate and maximum biomass concentration of Clostridium ljungdahlii are unaffected by cyanide at concentrations of up to 1.0 mM with fructose as a carbon source and up to 0.1 mM with syngas as a carbon source. After the culture is adapted to cyanide it shows no growth inhibition. While the difference in growth is an increasing lag-phase with increasing cyanide concentrations, the product spectrum shifts from 97% acetic acid and 3% ethanol at 0 mM cyanide to 20% acetic acid and 80% ethanol at 1.0 mM cyanide for cultures growing on (fructose) and 80% acetic acid and 20% ethanol at 0.1 mM cyanide (syngas).
Keywords: Clostridium ljungdahlii; crude syngas; cyanide; ethanol formation; growth inhibition; syngas fermentation; syngas impurities.
Figures

References
-
- Anderson M. E., DeRose V. J., Hoffman B. M., Lindahl P. A. (1993). Identification of a cyanide binding site in CO dehydrogenase from Clostridium thermoaceticum using EPR and ENDOR spectroscopies. J. Am. Chem. Soc. 115 12204–12205. 10.1021/ja00078a085 - DOI
-
- Boerrighter H., den Uil H., Calis H.-P. (2013). “Green diesel from biomass via Fischer-Tropsch synthesis: new insights in gas cleaning and process design,” in Pyrolysis and Gasification of Biomass and Waste ed. Bridgwater A. V. (Newbury: CPL Press; ) 371–383.
-
- Broer K. M., Woolock P. J., Johnston P. A., Brown R. C. (2015). Steam/oxygen gasification system for the production of clean syngas. Fuel 140 282–292. 10.1016/j.fuel.2014.09.078 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

