Geranylgeraniol Prevents Statin-Dependent Myotoxicity in C2C12 Muscle Cells through RAP1 GTPase Prenylation and Cytoprotective Autophagy
- PMID: 29951166
- PMCID: PMC5987243
- DOI: 10.1155/2018/6463807
Geranylgeraniol Prevents Statin-Dependent Myotoxicity in C2C12 Muscle Cells through RAP1 GTPase Prenylation and Cytoprotective Autophagy
Abstract
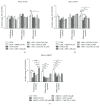
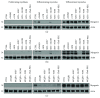
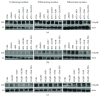
The present study investigated the cytotoxic effects of statins (atorvastatin (ATR) and simvastatin (SIM), resp.) and methyl-beta-cyclodextrin (MβCD), at their respective IC50 concentrations, on muscle regeneration in the in vitro model of murine C2C12 myoblasts. Cotreatment with mevalonate (MEV), farnesol (FOH), geranylgeraniol (GGOH), or water-soluble cholesterol (Chol-PEG) was employed to determine whether the statin-dependent myotoxicity resulted from the lower cholesterol levels or the attenuated synthesis of intermediates of mevalonate pathway. Our findings demonstrated that while GGOH fully reverted the statin-mediated cell viability in proliferating myoblasts, Chol-PEG exclusively rescued MβCD-induced toxicity in myocytes. Statins caused loss of prenylated RAP1, whereas the GGOH-dependent positive effect was accompanied by loss of nonprenylated RAP1. Geranylgeranyltransferases are essential for muscle cell survival as inhibition with GGTI-286 could not be reversed by GGOH cotreatment. The increase in cell viability correlated with elevated AKT 1(S463) and GSK-3β(S9) phosphorylations. Slight increase in the levels of autophagy markers (Beclin 1, MAP LC-3IIb) was found in response to GGOH cotreatment. Autophagy rose time-dependently during myogenesis and was inhibited by statins and MβCD. Statins and MβCD also suppressed myogenesis and neither nonsterol isoprenoids nor Chol-PEG could reverse this effect. These results point to GGOH as the principal target of statin-dependent myotoxicity, whereas plasma membrane cholesterol deposit is ultimately essential to restore viability of MβCD-treated myocytes. Overall, this study unveils for the first time a link found between the GGOH- and Chol-PEG-dependent reversal of statin- or MβCD-mediated myotoxicity and cytoprotective autophagy, respectively.
Figures






References
-
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. Journal of Lipid Research. 1992;33(11):1569–1582. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

