Dexamethasone implant in silicone oil: in vitro behavior
- PMID: 29951249
- PMCID: PMC6011200
- DOI: 10.1186/s40942-018-0127-x
Dexamethasone implant in silicone oil: in vitro behavior
Abstract
Background: To determine the effect of the silicone on the dexamethasone intravitreal implant.
Methods: Basic, experimental, prospective and transversal study performed at the hospital "Nuestra Señora de la Luz" in Mexico City. One dexamethasone implant was placed in a test tube with 4 mL of each tamponade medium: 1000cS, 5000cS and heavy silicone oil; basic saline solution was used as the control medium. Photographs were taken weekly for 12 months. 200 µL samples were taken from each medium at 24 h, 1, 2 weeks and monthly for 12 months. ELISA test was performed to quantify dexamethasone release in every sample. An inflammatory stimulus was created and later exposed it to every sample in order to test their anti-inflammatory capacity by cytokine analysis using cytometric bead array. Statistically significant results were obtained with p < 0.05.
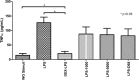
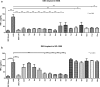
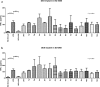
Results: Photographic follow-up showed disintegration of the implant in control medium. Implants in silicone oil suffered no changes during follow-up. Dexamethasone levels in control medium showed stability from month 2 to 12. Silicone oil mediums showed irregular dexamethasone release during the 1 year period. Dexamethasone in control medium had inhibitory effects on TNF-α starting at 24 h (p < 0.001) and remained stable. Dexamethasone in 1000cS silicone oil showed inhibitory effects from month 2 (p < 0.001) until month 6 (p < 0.001). Implants in denser silicone oils showed no inhibitory effects in any of the samples.
Conclusions: Denser mediums altered the implant pharmacokinetics and showed no anti-inflammatory effects even when concentrations were quantified at levels similar to control medium in vitro.
Keywords: Dexamethasone; Silicone oil; Tamponade.
Figures



References
-
- Liquid silicone for complex retinal detachments. Lancet. 1991; 337(8737):332–3. - PubMed
-
- Scott JD. Silicone oil as an instrument. In: Ryan S, editor. Retina. St Louis: Mosby; 1989.
LinkOut - more resources
Full Text Sources
Other Literature Sources

