Do immune checkpoint inhibitors need new studies methodology?
- PMID: 29951307
- PMCID: PMC5994495
- DOI: 10.21037/jtd.2018.01.131
Do immune checkpoint inhibitors need new studies methodology?
Abstract
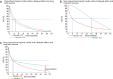
Immune checkpoint inhibitors (ICI) have widely reshaped the treatment paradigm of advanced cancer patients. Although multiple studies are currently evaluating these drugs as monotherapies or in combination, the choice of the most accurate statistical methods, endpoints and clinical trial designs to estimate the benefit of ICI remains an unsolved methodological issue. Considering the unconventional patterns of response or progression [i.e., pseudoprogression, hyperprogression (HPD)] observed with ICI, the application in clinical trials of novel response assessment tools (i.e., iRECIST) able to capture delayed benefit of immunotherapies and/or to quantify tumor dynamics and kinetics over time is an unmet clinical need. In addition, the proportional hazard model and the conventional measures of survival [i.e., median overall or progression free survival (PFS) and hazard ratios (HR)] might usually result inadequate in the estimation of the long-term benefit observed with ICI. For this reason, innovative methodologies such as milestone analysis, restricted mean survival time (RMST), parametric models (i.e., Weibull distribution, weighted log rank test), should be systematically investigated in clinical trials in order to adequately quantify the fraction of patients who are "cured", represented by the tails of the survival curves. Regarding predictive biomarkers, in particular PD-L1 expression, the integration and harmonization of the existing assays are urgently needed to provide clinicians with reliable diagnostic tests and to improve patient selection for immunotherapy. Finally, developing original and high-quality study designs, such as adaptive or basket biomarker enriched clinical trials, included in large collaborative platforms with multiple active sites and cross-sector collaboration, represents the successful strategy to optimally assess the benefit of ICI in the next future.
Keywords: Immune checkpoint inhibitors (ICI); clinical trial design; long-term benefit; milestone; survival analysis.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
