Identifying a Clinically Applicable Mutational Burden Threshold as a Potential Biomarker of Response to Immune Checkpoint Therapy in Solid Tumors
- PMID: 29951597
- PMCID: PMC6016848
- DOI: 10.1200/PO.17.00146
Identifying a Clinically Applicable Mutational Burden Threshold as a Potential Biomarker of Response to Immune Checkpoint Therapy in Solid Tumors
Abstract
Purpose: An association between mutational burden and response to immune checkpoint therapy has been documented in several cancer types. The potential for such a mutational burden threshold to predict response to immune checkpoint therapy was evaluated in several clinical datasets, where mutational burden was measured either by whole-exome sequencing (WXS) or using commercially available sequencing panels.
Methods: WXS and RNA-seq data of 33 solid cancer types from TCGA were analyzed to determine whether a robust immune checkpoint activating mutation (iCAM) burden threshold associated with evidence of immune checkpoint activation exists in these cancers that may serve as a biomarker for response to immune checkpoint blockade therapy.
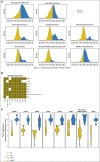
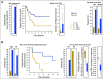
Results: We find that a robust iCAM threshold, associated with signatures of immune checkpoint activation, exists in 8 of 33 solid cancers: melanoma, lung adenocarcinoma, colon adenocarcinoma, endometrial cancer, stomach adenocarcinoma, cervical cancer, ER+HER2- breast cancer, and bladder-urothelial cancer. Tumors with mutational burden higher than the threshold (iCAM+) also had clear histologic evidence of lymphocytic infiltration. In published datasets of melanoma, lung adenocarcinoma and colon cancer, patients with iCAM+ tumors had significantly better response to immune checkpoint therapy compared to those with iCAM- tumors. ROC analysis using TCGA predictions as gold standard showed that iCAM+ tumors are accurately identifiable using clinical sequencing assays, such as FoundationOne or StrandAdvantage. Using the FoundationOne derived threshold, analysis of 113 melanoma tumors, showed that iCAM+ patients have significantly better response to immune checkpoint therapy. iCAM+ and iCAM- tumors have distinct mutation patterns and different immune microenvironments.
Conclusion: In 8 solid cancers, a mutational burden threshold exists that may predict response to immune checkpoint blockade. This threshold is identifiable using available clinical sequencing assays.
Conflict of interest statement
Authors' Disclosures of Potential Conflicts of Interest: Anshuman Panda, No relationship to disclose Anil Betigeri, Employment: Strand Life Sciences Kalyanasundaram Subramanian, Employment: Strand Life Sciences, Syngene International, Patents, Royalties, Other Intellectual Property: Patent to predict drug toxicity Jeffrey S. Ross, Leadership: Foundation Medicine, Employment: Foundation Medicine, Stock and Other Ownership Interests: Foundation Medicine Dean C. Pavlick, Employment: Foundation Medicine, Stock and Other Ownership Interests: Foundation Medicine Siraj Ali, Employment: Foundation Medicine, Stock and Other Ownership Interests: Exelixis, Blueprint Medicines, Agios, Patents, Royalties, Other Intellectual Property: Patents via Foundation Medicine, patents via Seres Health on microbiome stuff in non-neoplastic disease (I) Paul Markowski, No relationship to disclose Ann Silk, Research Funding: Amgen (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Paometheus (Inst), Viralytics (Inst) Howard L. Kaufman, Employment: Compass Therapeutics, Leadership: Compass Therapeutics, Honoraria: Amgen, EMD Serono, Merck, Celldex, Prometheus, Turnstone Bio, Compass Therapeutic, Consulting or Advisory Role: Amgen, Merck, Merck Serono, Paometheus, Celldex, Turnstone Bio, Bristol-Myers Squibb, Compass Therapeutics, Speakers' Bureau: Merck, Research Funding: Amgen (Inst), Merck (Inst), Travel, Accommodations, Expenses: EMD Serono, Turnstone Bio Edmund Lattime, Stock and Other Ownership Interests: Johnson & Johnson (I), Patents, Royalties, Other Intellectual Property: I am an inventor of an oncolytic virus, for which the patent is held by, Thomas Jefferson University, where I worked from 1989 to 1998. The patent is licensed by Silagen. I receive a small annual fee from Thomas Jefferson University. Janice M. Mehnert, Honoraria: Genentech, EMD Serono, Consulting or Advisory Role: Merck Sharp & Dohme, Amgen, Research Funding: Merck (Inst), Sanofi (Inst), Novartis (Inst), Polynoma (Inst), Immunocore (Inst), Amgen (Inst),, AstraZeneca (Inst), Travel, Accommodations, Expenses: EMD Serono, Merck Sharp & Dohme, Other Relationship: Amgen, EMD Serono, Merck Ryan Sullivan, Honoraria: Genentech, Consulting or Advisory Role: Novartis, Biodesix, Paometheus, Amgen, Takeda, WorldCare Clinical, ACI Clinical, Merck, BioLineRx, Research Funding: Amgen (Inst), Eli Lilly (Inst), BioMed Valley Discoveries (Inst), Merck (Inst), Deciphera (Inst),, Genentech (Inst), Other Relationship: Boehringer Ingelheim Christine M. Lovly, Honoraria: Novartis, Sequenom, Qiagen, Pfizer, NCCN, Takeda, Consulting or Advisory Role: ARIAD, Clovis Oncology, Genoptix, Novartis, Foundation Medicine, Research Funding: AstraZeneca, Novartis, Travel, Accommodations, Expenses: Pfizer Jeffrey Sosman, Honoraria: Amgen, Merck, Array BioPharma, Bristol-Myers Squibb, Consulting or Advisory Role: Amgen, Merck, Array BioPharma, Bristol-Myers Squibb Douglas B. Johnson, Consulting or Advisory Role: Bristol-Myers Squibb, Genoptix, Merck, Novartis, Incyte, Research Funding: Incyte, Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy Gyan Bhanot, No relationship to disclose
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

