Role of pannexin 1 channels in load-induced skeletal response
- PMID: 29952014
- PMCID: PMC6310681
- DOI: 10.1111/nyas.13914
Role of pannexin 1 channels in load-induced skeletal response
Abstract
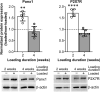
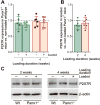
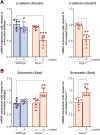
The pannexin 1 (Panx1) channel is a mechanosensitive channel that interacts with P2X7 receptors (P2X7R) to form a functional complex that has been shown in vitro to play an essential role in osteocyte mechanosignaling. While the participation of P2X7R in skeletal responses to mechanical loading has been demonstrated, the role of Panx1 and its interplay with P2X7R still remain to be determined. In this study, we use a global Panx1-/- mouse model and in vivo mechanical loading to demonstrate that Panx1 channels play an essential role in load-induced skeletal responses. We found that absence of Panx1 not only disrupts the P2X7R-Panx1 signaling complex, but also alters load-induced regulation of P2X7R expression. Moreover, lack of Panx1 completely abolished load-induced periosteal bone formation. Load-induced regulation of β-catenin and sclerostin expression was dysregulated in Panx1-/- , compared to wild-type, bone. This finding suggests that Panx1 deficiency disrupts Wnt/β-catenin signaling by lowering β-catenin while favoring inhibition of bone formation by increasing load-induced sclerostin expression. This study demonstrates the existence of a Panx1-dependent mechanosensitive mechanism that not only modulates ATP signaling but also coordinates Wnt/β-catenin signaling that is essential for proper skeletal response to mechanical loading.
Keywords: Panx1; mechanical loading; osteocytes; sclerostin; β-catenin.
© 2018 New York Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
P2X7R-Panx1 Complex Impairs Bone Mechanosignaling under High Glucose Levels Associated with Type-1 Diabetes.PLoS One. 2016 May 9;11(5):e0155107. doi: 10.1371/journal.pone.0155107. eCollection 2016. PLoS One. 2016. PMID: 27159053 Free PMC article.
-
Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes.J Bone Miner Res. 2016 Apr;31(4):890-9. doi: 10.1002/jbmr.2740. Epub 2016 Jan 20. J Bone Miner Res. 2016. PMID: 26553756 Free PMC article.
-
Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling.PLoS One. 2014 Aug 29;9(8):e106269. doi: 10.1371/journal.pone.0106269. eCollection 2014. PLoS One. 2014. PMID: 25170954 Free PMC article.
-
Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain.Pharmacol Res. 2015 Nov;101:86-93. doi: 10.1016/j.phrs.2015.07.016. Epub 2015 Jul 23. Pharmacol Res. 2015. PMID: 26211949 Review.
-
Pannexin-1 channels in epilepsy.Neurosci Lett. 2019 Mar 16;695:71-75. doi: 10.1016/j.neulet.2017.09.004. Epub 2017 Sep 5. Neurosci Lett. 2019. PMID: 28886985 Review.
Cited by
-
Mechanotransduction via the coordinated actions of integrins, PI3K signaling and Connexin hemichannels.Bone Res. 2021 Feb 2;9(1):8. doi: 10.1038/s41413-020-00126-w. Bone Res. 2021. PMID: 33531460 Free PMC article.
-
ATP transporters in the joints.Purinergic Signal. 2021 Dec;17(4):591-605. doi: 10.1007/s11302-021-09810-w. Epub 2021 Aug 15. Purinergic Signal. 2021. PMID: 34392490 Free PMC article. Review.
-
Inhibition of Pannexin 1 Reduces the Tumorigenic Properties of Human Melanoma Cells.Cancers (Basel). 2019 Jan 16;11(1):102. doi: 10.3390/cancers11010102. Cancers (Basel). 2019. PMID: 30654593 Free PMC article.
-
The Impact of Plasma Membrane Ion Channels on Bone Remodeling in Response to Mechanical Stress, Oxidative Imbalance, and Acidosis.Antioxidants (Basel). 2023 Mar 10;12(3):689. doi: 10.3390/antiox12030689. Antioxidants (Basel). 2023. PMID: 36978936 Free PMC article. Review.
-
Purinergic P2 Receptors: Novel Mediators of Mechanotransduction.Front Pharmacol. 2021 May 7;12:671809. doi: 10.3389/fphar.2021.671809. eCollection 2021. Front Pharmacol. 2021. PMID: 34025431 Free PMC article. Review.
References
-
- Robling AG, Hinant FM, Burr DB, et al. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 2002;17:1545–1554. - PubMed
-
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annual review of biomedical engineering. 2006;8:455–498. - PubMed
-
- Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J. Cell. Biochem. 1994;55:287–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

