ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation
- PMID: 29952768
- PMCID: PMC6118586
- DOI: 10.1172/JCI96519
ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation
Abstract
DNA-damaging chemotherapy and radiation therapy are integrated into the treatment paradigm of the majority of cancer patients. Recently, immunotherapy that targets the immunosuppressive interaction between programmed death 1 (PD-1) and its ligand PD-L1 has been approved for malignancies including non-small cell lung cancer, melanoma, and head and neck squamous cell carcinoma. ATR is a DNA damage-signaling kinase activated at damaged replication forks, and ATR kinase inhibitors potentiate the cytotoxicity of DNA-damaging chemotherapies. We show here that the ATR kinase inhibitor AZD6738 combines with conformal radiation therapy to attenuate radiation-induced CD8+ T cell exhaustion and potentiate CD8+ T cell activity in mouse models of Kras-mutant cancer. Mechanistically, AZD6738 blocks radiation-induced PD-L1 upregulation on tumor cells and dramatically decreases the number of tumor-infiltrating Tregs. Remarkably, AZD6738 combines with conformal radiation therapy to generate immunologic memory in complete responder mice. Our work raises the possibility that a single pharmacologic agent may enhance the cytotoxic effects of radiation while concurrently potentiating radiation-induced antitumor immune responses.
Keywords: Cancer; Immunology; Oncology.
Conflict of interest statement
Figures
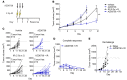






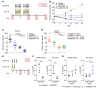
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

