Methods for 2-Deoxyglycoside Synthesis
- PMID: 29953219
- PMCID: PMC6135715
- DOI: 10.1021/acs.chemrev.7b00731
Methods for 2-Deoxyglycoside Synthesis
Abstract
Deoxy-sugars often play a critical role in modulating the potency of many bioactive natural products. Accordingly, there has been sustained interest in methods for their synthesis over the past several decades. The focus of much of this work has been on developing new glycosylation reactions that permit the mild and selective construction of deoxyglycosides. This Review covers classical approaches to deoxyglycoside synthesis, as well as more recently developed chemistry that aims to control the selectivity of the reaction through rational design of the promoter. Where relevant, the application of this chemistry to natural product synthesis will also be described.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








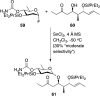
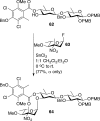

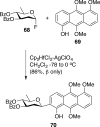
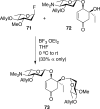





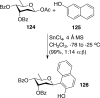

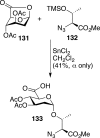


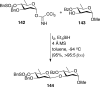
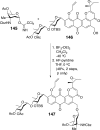







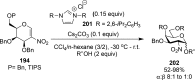

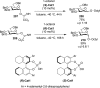
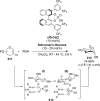
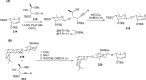
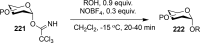
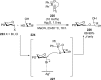
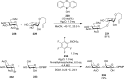

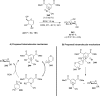







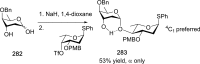
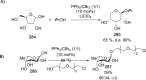



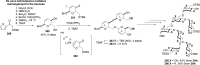
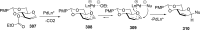

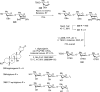
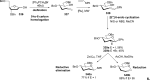
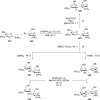
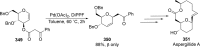



References
-
- Marzabadi C. H.; Franck R. W. The Synthesis of 2-Deoxyglycosides: 1988–1999. Tetrahedron 2000, 56, 8385–8417. 10.1016/S0040-4020(00)00691-8. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

