Immunotherapy of Guillain-Barré syndrome
- PMID: 29953326
- PMCID: PMC6314401
- DOI: 10.1080/21645515.2018.1493415
Immunotherapy of Guillain-Barré syndrome
Abstract
Guillain-Barré syndrome (GBS), the most common cause of acute neuromuscular weakness and paralysis worldwide, encompasses a group of acute immune-mediated disorders restricted to peripheral nerves and roots. Immune-mediated attack of peripheral nervous system myelin, axons or both is presumed to be triggered by molecular mimicry, with both cell- and humoral-dependent mechanisms implicated in disease pathogenesis. Good circumstantial evidence exists for a pathogenic role for molecular mimicry in GBS pathogenesis, especially with its axonal forms, providing insights that could guide future immunotherapy. Intravenous immunoglobulin (IVIg) and plasma exchange (PE) are the most commonly prescribed immunotherapies for GBS with variable efficacy dependent on GBS subtype, severity at initial presentation and other clinical and electrophysiologic prognostic factors. The mechanisms of action of IVIg and PE are not known definitely. Despite recent significant advances in molecular biology that provide insights into GBS pathogenesis, no advances in therapeutics or significant improvements in patient outcomes have occurred over the past three decades. We summarize the clinical aspects of GBS, its current pathogenesis and immunotherapy, and highlight the potential of leukocyte trafficking inhibitors as novel disease-specific immunotherapeutic drugs.
Keywords: Blood-Nerve Barrier; Guillain-Barré syndrome; Immunopathology; Immunotherapy; Leukocyte Trafficking; Peripheral Nerve Inflammation.
Figures

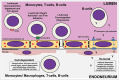
References
-
- Guillain G, Barre JA, Strohl A. [Radiculoneuritis syndrome with hyperalbuminosis of cerebrospinal fluid without cellular reaction. Notes on clinical features and graphs of tendon reflexes. 1916]. Ann Med Interne (Paris). 1999;150:24–32. - PubMed
-
- A prospective study on the incidence and prognosis of Guillain-Barre syndrome in Emilia-Romagna region, Italy (1992-1993) Emilia-Romagna Study Group on Clinical and Epidemiological Problems in Neurology. Neurology. 1997; 48:214–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
